��Ŀ����
(1)��ѧƽ�ⳣ��K��ʾ���淴Ӧ�Ľ��г̶ȣ�KֵԽ��ʾ���淴Ӧ���е�Խ��ȫ��Kֵ��С���¶ȵĹ�ϵ�ǣ��¶����ߣ�Kֵ (�һ��������һ����С����������Ҳ���ܼ�С��)��
(2)��һ���Ϊ10 L�������У�ͨ��һ������CO��H2O����800��ʱ�������·�Ӧ��CO(g)+H2O(g) CO2(g)+H2(g)��H��0��CO��H2O�����ʵ���Ũ�ȱ仯��ͼ��ʾ����
CO2(g)+H2(g)��H��0��CO��H2O�����ʵ���Ũ�ȱ仯��ͼ��ʾ����
��0��4 minʱ���ƽ����Ӧ����v(CO)�� mol��L-1��min-1��
����800��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��K�� (Ҫ��д������ʽ����ֵ)��CO��ת���ʣ� ��
����800��ʱ������Ӧ��ʼʱ��������CO��H2O��Ũ�ȷֱ�Ϊ0.20 mol��L-1��0.80 mol��L-1����ﵽƽ��ʱCOת��ΪCO2��ת������ ��
(1)��������Ҳ���ܼ�С
(2)��0.03 ��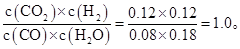 60% ��80%
60% ��80%
����

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�һ�������������Ժ�CO2������Ӧ��Fe(s)��CO2(g) FeO(s)��CO(g)��H��0��1100��ʱ����ij�ܱ������м����������۲�����һ������CO2���壬��Ӧ������CO2�����CO�����Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)��CO(g)��H��0��1100��ʱ����ij�ܱ������м����������۲�����һ������CO2���壬��Ӧ������CO2�����CO�����Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��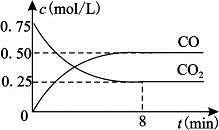
��1�����д�ʩ����ʹƽ��ʱK�������___________������ţ���
A�������¶� B������ѹǿC������һ����COD�������¶�
��2��8�����ڣ�CO��ƽ����Ӧ����v��CO��=___________mol/(L��min)��
��3��1100��ʱ��2L���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������
| ���� | �� | �� |
| ��Ӧ��Ͷ���� | 3molFe��2molCO2 | 4molFeO��3molCO |
| CO��Ũ��(mol/L) | C1 | C2 |
| CO2��������� | 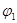 | 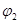 |
| ��ϵѹǿ��Pa�� | P1 | P2 |
| ��̬��Ӧ���ת���� | 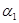 | 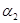 |
A��
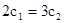 B��
B��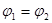 C��P1��P2D��
C��P1��P2D��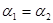
����
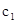 ��___________��
��___________��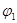 ��___________��
��___________��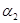 ��___________��
��___________�� ��4�֣���һ���Ϊ10L�ܱյ������У�ͨ��һ������CO��H2O��g������850��ʱ�������·�Ӧ��CO(g)+H2O(g)  CO2(g)+H2(g) ��H��0
CO2(g)+H2(g) ��H��0
��1��CO��H2OŨ�ȱ仯��ͼ����0��4 min��ƽ����Ӧ���ʦ�(CO)��_______ mol/��L��min������ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ �� 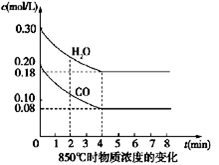
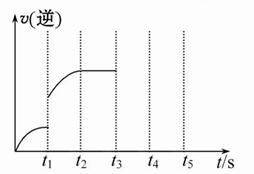
��2����������Щ���������ٷ����仯ʱ������������Ӧ�Ѵﵽƽ��״̬���� ��
| A����������ѹǿ |
| B�����������ܶ� |
| C��CO�����ʵ���Ũ�� |
| D���ܱ������зų����� |
��6�֣��������ƣ�NaClO2����һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ�����ڼ��Ի������ȶ����ڡ�ijͬѧ�������Ϻ��������NaClO2����Ҫ�������¡�
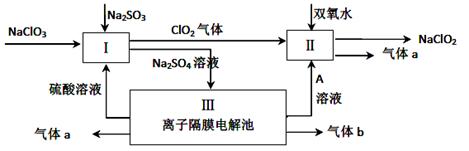
��3����ƽ���з�Ӧ����ʽ ClO3-+ H++ SO32-== ClO2��+ SO42-+
��4��A�Ļ�ѧʽ�� ������������a�ĵ缫��Ӧʽ ��
 CO2(g)��H2(g)����Ӧ�ų���������Ӧ��CO2��Ũ����ʱ��仯�������ͼ��ʾ��
CO2(g)��H2(g)����Ӧ�ų���������Ӧ��CO2��Ũ����ʱ��仯�������ͼ��ʾ��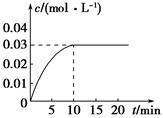
 H2��+I2
H2��+I2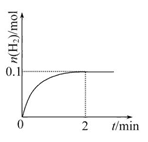
 CO(g)��3H2(g)����H��206.2 kJ��mol��1��Kp��________�������¶ȵ����ߣ���ƽ�ⳣ��________(�������С�����䡱)��
CO(g)��3H2(g)����H��206.2 kJ��mol��1��Kp��________�������¶ȵ����ߣ���ƽ�ⳣ��________(�������С�����䡱)�� W(s)��3H2O(g)�Ļ�ѧƽ�ⳣ������ʽΪ________��
W(s)��3H2O(g)�Ļ�ѧƽ�ⳣ������ʽΪ________�� yC(g)����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
yC(g)����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺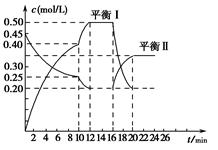
 CaSO4��2H2O(s)+2 OH��
CaSO4��2H2O(s)+2 OH��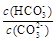 ��С
��С