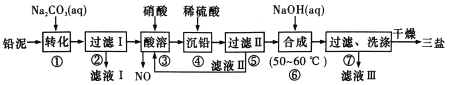
��Ŀ����
����Ŀ�����ǵ�IIIA��Ԫ�أ��������ڼ���������������ַǽ�����Ӧ��ijͬѧ�����������͵�����Ӧ�Ʊ����Ȼ�����֪BCl3�ķе�Ϊ12.5�棬�۵�Ϊ-107.3�棬��ˮ���ҷ�Ӧ��
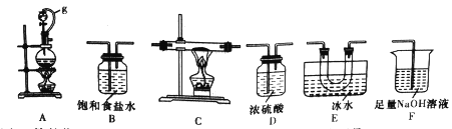
��1��ѡ����ͼ��ʾ��װ�ã������ظ�ѡ�ã�����ʵ�飬װ���������ӵĺ���˳��Ϊ________��
��2��ͼ��g�ܵ�������______��װ��E��������_______��
��3����ʼʵ��ʱ���ȵ�ȼ____(�A����B��)���ľƾ��ơ�
��4����д��BCl3��ˮ���ʵĻ�ѧ����ʽ___________��
��5��������һԪ���ᣬ�����λ�ѧʽΪNa[B(OH)4]����������ˮ�е��뷽��ʽ��______��
��6��ʵ����ɺ�ijͬѧ��F (��Һ�к���0.05 mol/LNaC10��0.05 mol/LNaCl��0.1 mol/L NaOH���еμ�Ʒ����Һ��������Һ��ɫ�������ʵ��̽����Һ��ɫ��ԭ���뽫�������ݲ������������ʵ�鷽����
ʵ����� | 0.1mol/LNaClO��Һ/mL | 0.1mol/LNaCl��Һ/mL | 0.2mol/LNaOH��Һ/mL | H2O /mL | Ʒ����Һ | ���� |
�� | 4.0 | 0 | 0 | x | 3�� | �Ͽ���ɫ |
�� | 0 | 4.0 | 4.0 | 0 | 3�� | ����ɫ |
�� | 4.0 | 0 | 4.0 | 0 | 3�� | ������ɫ |
x=_______�����ۣ�_______________��
���𰸡� ABDCEDF ������ѹƽ�⣬���ڷ�Һ©���е�Һ������������ƿ �������ռ�BCl3 A BCl3��3H2O=H3BO3��3HCl H3BO3��H2O![]() [B(OH)4]-��H+ 4.0 NaClOʹƷ����Һ��ɫ����Һ����Խǿ��ɫԽ����
[B(OH)4]-��H+ 4.0 NaClOʹƷ����Һ��ɫ����Һ����Խǿ��ɫԽ����
����������1�������������͵�����Ӧ�Ʊ����Ȼ�����֪�������ڼ���������������ַǽ�����Ӧ��BCl3�ķе�Ϊ12.5�棬�۵�Ϊ-107.3�棬��ˮ���ҷ�Ӧ����������Ʊ�����ྻ�����������ҷ�Ӧ�����б��з�ֹˮ�������У���AΪ��ȡ����װ�ã�Ȼ����������ͨ��Bװ���Գ�ȥ�����е��Ȼ��⣬��ͨ��Dװ�ø��������ͨ��Cװ���м�������������Ӧ�����������Ȼ����������Eװ����ȴ�ռ�������������Dװ�ø����ֹ���ˮ�������У��������F����β����������˽�����˳��Ϊ��ABDCEDF����2��ͼ��g�ܵ���������������ѹƽ�⣬���ڷ�Һ©���е�Һ������������ƿ��װ��E�����������������ռ�BCl3����3����ʼʵ��ʱ���ȵ�ȼA���ľƾ�����ȡ������ʹ���淴Ӧװ���г����������������4����д��BCl3��ˮˮ������������ἰ���ᣬ�仯ѧ����ʽ��BCl3��3H2O=H3BO3��3HCl����5��������һԪ���ᣬ�����λ�ѧʽΪNa[B(OH)4]����������ˮ�е��뷽��ʽ��H3BO3��H2O![]() [B(OH)4]-��H+����6�����ʵ��̽����ͬ��Һ��Ʒ����ɫ��ԭ��ͨ����Һ��Ũ�Ȳ�ͬ����Һ���������ͬ�����ݢڢۿ�֪����Һ�����Ϊ8.0mL����x=4.0mL��ʵ��٢���NaClO����ɫ��ʵ���û��NaClO����ɫ��ʵ�����ʵ��۶Աȣ����������£���ɫ���������Ϊ��NaClOʹƷ����Һ��ɫ����Һ����Խǿ��ɫԽ����
[B(OH)4]-��H+����6�����ʵ��̽����ͬ��Һ��Ʒ����ɫ��ԭ��ͨ����Һ��Ũ�Ȳ�ͬ����Һ���������ͬ�����ݢڢۿ�֪����Һ�����Ϊ8.0mL����x=4.0mL��ʵ��٢���NaClO����ɫ��ʵ���û��NaClO����ɫ��ʵ�����ʵ��۶Աȣ����������£���ɫ���������Ϊ��NaClOʹƷ����Һ��ɫ����Һ����Խǿ��ɫԽ����