��Ŀ����
Ϊ��֤��CO2��Na2O2��O2����,�������������ʵ��װ��(��ͼ)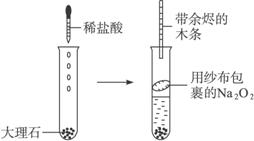
������������:�����Թ��з��뼸�����ʯ,����2��3 mLϡ����
����ȼ�ŵĻ�����CO2������
�������ӽ���ɴ�����õ�Na2O2�����Թ��в�
���ô������ľ������O2�������������������:
(1)Ϊʲô�ڰ���Na2O2��ɴ������֮ǰ�����û����飿
(2)ʵ��ܵ�����������
(3)���ʵ��ڢܲ�δ�۲쵽Ӧ����������ܵ�ԭ����ʲô��
(1)ȷ֤O2����CO2��Na2O2��Ӧ������ġ�
(2)�������ľ����ȼ��
(3)A.Na2O2����̫�٣�B.����Ũ�ȹ�����ʹCO2����̫�㣻C.�������ľ�����벻��ʱ��

��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ
 ijУ��ѧ�о���ѧϰС��Ϊ��֤��ͭ��ϡ���ᷴӦ����һ������������ͼ��ʾװ�ý���ʵ�飬������װ�úͼг�װ�þ�����ȥ���������Ѽ��飬F�����ڹ��������˫��������
ijУ��ѧ�о���ѧϰС��Ϊ��֤��ͭ��ϡ���ᷴӦ����һ������������ͼ��ʾװ�ý���ʵ�飬������װ�úͼг�װ�þ�����ȥ���������Ѽ��飬F�����ڹ��������˫��������
