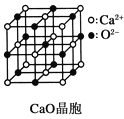
��Ŀ����
����Ŀ��ͨ�����·�Ӧ�ɻ��������Դ��������CH3OCH3)������˵������ȷ����
��C(s) + H2O(g)==CO(g)+H2(g) ��H1=a kJmol-1
��CO(g) + H2O(g)==CO2(g)+H2(g) ��H2=b kJmol-1
��CO2(g)+3H2(g)==CH3OH(g)+H2O(g) ��H3 =c kJmol-1
��2CH3OH(g) ==CH3OCH3(g)+H2O(g) ��H4=d kJmol-1
A. ��Ӧ�٢�Ϊ��Ӧ���ṩԭ����
B. ��Ӧ��Ҳ��CO2��Դ�����õķ���֮һ
C. ��ӦCH3OH(g)== ![]() CH3OCH3(g)+
CH3OCH3(g)+ ![]() H2O(1)����H =
H2O(1)����H =![]() kJmol-1
kJmol-1
D. ��Ӧ 2CO(g)+4H2(g) ==CH3OCH3(g)+H2O(g)����H= (2b+2c+d) kJmol-1
���𰸡�C
��������A����Ӧ�١��ڵ�������CO2��H2�Ƿ�Ӧ�۵ķ�Ӧ�ѡ��A��ȷ��B����Ӧ�ۿɽ�������̼ת��Ϊ�״������Ϊ����ѡ��B��ȷ��C��4����Ӧ�У�ˮȫ����̬��û�и���ˮ����̬��ΪҺ̬���ʱ䣬ѡ��C����D���ѷ�Ӧ�ڢۢ�������Ӧ��(��+��)2+�ܿɵø÷�Ӧ����Ӧ���ʱ䣬ѡ��D��ȷ����ѡC��

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�����Ŀ��NH3��һ����Ҫ�Ļ���ԭ�ϣ�����������������;�㷺��
��1����֪��
���ۼ� | ����/ kJ��mol-1 |
H�DH | 436 |
N��N | 946 |
N�DH | 391 |
ע������̬������1 molij�ֹ��ۼ���Ҫ���յ����������Ǹù��ۼ��ļ��ܡ�
N2 (g)��3 H2 (g)![]() 2 NH3 (g) H =____kJ��mol-1
2 NH3 (g) H =____kJ��mol-1
��2��һ���¶��£�����ݵ��ܱ������г���N2��H2������Ӧ��N2 ��3H2 ![]() 2NH3����ø����Ũ����ʱ��仯��ͼ1��ʾ��
2NH3����ø����Ũ����ʱ��仯��ͼ1��ʾ��

�ٱ�ʾc(N2)��������__�������A����������B��������C������
��0��t0ʱ��H2��ʾ��Ӧ����v(H2)____mol��L-1��min-1��
��������˵���÷�Ӧ�ﵽƽ�����____��
a����������ѹǿ���ٱ仯
b��2c(H2)= 3c(NH3)
c�����������������ٱ仯
d��NH3������������ٱ仯
��3��DZͧ��ʹ�õ�Һ��-Һ��ȼ�ϵ�ع���ԭ����ͼ2��ʾ��

�ٵ缫b������____��
�ڵ������Һ��OH-������____�ƶ�����缫a���缫b������
�۵缫a�ĵ缫��ӦʽΪ____��
��4����ͨ��NH3��NaClO��Ӧ���Ƶû��ȼ���£�N2H4�����÷�Ӧ�Ļ�ѧ��Ӧ����ʽ��____��