��Ŀ����
��2013?��������ģ��X�� ���Ǻϳ�ij���������ϼ��ĵ��壬X�ĺϳ�·�����£�
���Ǻϳ�ij���������ϼ��ĵ��壬X�ĺϳ�·�����£�
��֪��
��ش��������⣺
��1���ɵ���X�ϳ����ø߷����ϼ��Ľṹ��ʽ��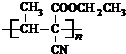
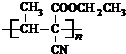 ��
��
��2������C�����������ŵ�������
��3��д����A��B��Ӧ�Ļ�ѧ����ʽ
��4��C��D��D��E�ķ�Ӧ˳���ܷ�ߵ���˵������
��5��C��D��A+F��G�ķ�Ӧ��������Ϊ
��6��F��ͬ���칹���У����������Һ��С�-C��C-���ṹ������CH3COOC��CH��HCOOC��CCH3�⣬���еĽṹ��ʽΪ
 ���Ǻϳ�ij���������ϼ��ĵ��壬X�ĺϳ�·�����£�
���Ǻϳ�ij���������ϼ��ĵ��壬X�ĺϳ�·�����£�
��֪��

��ش��������⣺
��1���ɵ���X�ϳ����ø߷����ϼ��Ľṹ��ʽ��
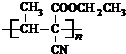
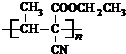
��2������C�����������ŵ�������
̼̼˫����ȩ��
̼̼˫����ȩ��
����3��д����A��B��Ӧ�Ļ�ѧ����ʽ
2CH3CH2OH+O2
2CH3CHO+2H2O
| Cu |
| �� |
2CH3CH2OH+O2
2CH3CHO+2H2O
��| Cu |
| �� |
��4��C��D��D��E�ķ�Ӧ˳���ܷ�ߵ���˵������
���ܵߵ���̼̼˫���ᱻ����
���ܵߵ���̼̼˫���ᱻ����
����5��C��D��A+F��G�ķ�Ӧ��������Ϊ
�ӳɷ�Ӧ
�ӳɷ�Ӧ
��������Ӧ
������Ӧ
����6��F��ͬ���칹���У����������Һ��С�-C��C-���ṹ������CH3COOC��CH��HCOOC��CCH3�⣬���еĽṹ��ʽΪ
HC��CCOOCH3��HCOOCH2C��CH
HC��CCOOCH3��HCOOCH2C��CH
����������G��HCN��Ӧ����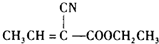 ����GΪCH3C��CCOOCH2CH3����F��A��Ӧ����CH3C��CCOOCH2CH3��CΪCH3CH=CHCHO�����巢���ӳɷ�Ӧ����DΪCH3CHBrCHBrCHO��D��������EΪCH3CHBrCBrCOOH��E������ȥ��Ӧ���ữ�õ�����FΪCH3C��CCOOH���ṹG�Ľṹ��֪��AΪCH3CH2OH��A��������������BΪCH3CHO������Ϣ�з�Ӧ��֪��ȩ�Ц�-H���ã�������ȩ�����ӳɷ�Ӧ����B��Cת����ϵ��֪����ȩ�ȷ����ӳɷ�Ӧ���ٷ���������ȥ��Ӧ����������C��CH3CH=CCHO�����ݴ˽��н��
����GΪCH3C��CCOOCH2CH3����F��A��Ӧ����CH3C��CCOOCH2CH3��CΪCH3CH=CHCHO�����巢���ӳɷ�Ӧ����DΪCH3CHBrCHBrCHO��D��������EΪCH3CHBrCBrCOOH��E������ȥ��Ӧ���ữ�õ�����FΪCH3C��CCOOH���ṹG�Ľṹ��֪��AΪCH3CH2OH��A��������������BΪCH3CHO������Ϣ�з�Ӧ��֪��ȩ�Ц�-H���ã�������ȩ�����ӳɷ�Ӧ����B��Cת����ϵ��֪����ȩ�ȷ����ӳɷ�Ӧ���ٷ���������ȥ��Ӧ����������C��CH3CH=CCHO�����ݴ˽��н��
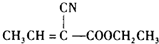 ����GΪCH3C��CCOOCH2CH3����F��A��Ӧ����CH3C��CCOOCH2CH3��CΪCH3CH=CHCHO�����巢���ӳɷ�Ӧ����DΪCH3CHBrCHBrCHO��D��������EΪCH3CHBrCBrCOOH��E������ȥ��Ӧ���ữ�õ�����FΪCH3C��CCOOH���ṹG�Ľṹ��֪��AΪCH3CH2OH��A��������������BΪCH3CHO������Ϣ�з�Ӧ��֪��ȩ�Ц�-H���ã�������ȩ�����ӳɷ�Ӧ����B��Cת����ϵ��֪����ȩ�ȷ����ӳɷ�Ӧ���ٷ���������ȥ��Ӧ����������C��CH3CH=CCHO�����ݴ˽��н��
����GΪCH3C��CCOOCH2CH3����F��A��Ӧ����CH3C��CCOOCH2CH3��CΪCH3CH=CHCHO�����巢���ӳɷ�Ӧ����DΪCH3CHBrCHBrCHO��D��������EΪCH3CHBrCBrCOOH��E������ȥ��Ӧ���ữ�õ�����FΪCH3C��CCOOH���ṹG�Ľṹ��֪��AΪCH3CH2OH��A��������������BΪCH3CHO������Ϣ�з�Ӧ��֪��ȩ�Ц�-H���ã�������ȩ�����ӳɷ�Ӧ����B��Cת����ϵ��֪����ȩ�ȷ����ӳɷ�Ӧ���ٷ���������ȥ��Ӧ����������C��CH3CH=CCHO�����ݴ˽��н������⣺��G��HCN��Ӧ����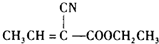 ����GΪCH3C��CCOOCH2CH3����F��A��Ӧ����CH3C��CCOOCH2CH3��CΪCH3CH=CHCHO�����巢���ӳɷ�Ӧ����DΪCH3CHBrCHBrCHO��D��������EΪCH3CHBrCBrCOOH��E������ȥ��Ӧ���ữ�õ�����FΪCH3C��CCOOH���ṹG�Ľṹ��֪��AΪCH3CH2OH��A��������������BΪCH3CHO������Ϣ�з�Ӧ��֪��ȩ�Ц�-H���ã�������ȩ�����ӳɷ�Ӧ����B��Cת����ϵ��֪����ȩ�ȷ����ӳɷ�Ӧ���ٷ���������ȥ��Ӧ����������C��CH3CH=CCHO����
����GΪCH3C��CCOOCH2CH3����F��A��Ӧ����CH3C��CCOOCH2CH3��CΪCH3CH=CHCHO�����巢���ӳɷ�Ӧ����DΪCH3CHBrCHBrCHO��D��������EΪCH3CHBrCBrCOOH��E������ȥ��Ӧ���ữ�õ�����FΪCH3C��CCOOH���ṹG�Ľṹ��֪��AΪCH3CH2OH��A��������������BΪCH3CHO������Ϣ�з�Ӧ��֪��ȩ�Ц�-H���ã�������ȩ�����ӳɷ�Ӧ����B��Cת����ϵ��֪����ȩ�ȷ����ӳɷ�Ӧ���ٷ���������ȥ��Ӧ����������C��CH3CH=CCHO����
��1��X����C=C˫���������Ӿ۷�Ӧ���ɸ߷���ճ�ϼ����ṹ��ʽΪ��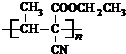 ���ʴ�Ϊ��
���ʴ�Ϊ��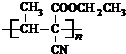 ��
��
��2��������������֪��CΪCH3CH=CCHO������̼̼˫����ȩ�����ʴ�Ϊ��̼̼˫����ȩ����
��3��A��B��Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2
2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��
��4������ȩ��Ϊ�Ȼ���̼̼˫���ᱻ������C��D��D��E�ķ�Ӧ˳���ܵߵ���
�ʴ�Ϊ�����ܵߵ���̼̼˫���ᱻ������
��4��C��DΪCH3CH=CCHO���巢���ӳɷ�Ӧ����CH3CHBrCBrCHO��
A+F��G��CH3CH2OH��CH3C��CCOOH����������Ӧ����CH3C��CCOOCH2CH3��
�ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��
��5��CH3C��CCOOH��ͬ���칹���У����������Һ��С�-C��C-���ṹ������CH3COOC��CH��HCOOC��CCH3��У�HC��CCOOCH3��HCOOCH2C��CH��
�ʴ�Ϊ��HC��CCOOCH3��HCOOCH2C��CH��
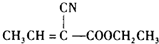 ����GΪCH3C��CCOOCH2CH3����F��A��Ӧ����CH3C��CCOOCH2CH3��CΪCH3CH=CHCHO�����巢���ӳɷ�Ӧ����DΪCH3CHBrCHBrCHO��D��������EΪCH3CHBrCBrCOOH��E������ȥ��Ӧ���ữ�õ�����FΪCH3C��CCOOH���ṹG�Ľṹ��֪��AΪCH3CH2OH��A��������������BΪCH3CHO������Ϣ�з�Ӧ��֪��ȩ�Ц�-H���ã�������ȩ�����ӳɷ�Ӧ����B��Cת����ϵ��֪����ȩ�ȷ����ӳɷ�Ӧ���ٷ���������ȥ��Ӧ����������C��CH3CH=CCHO����
����GΪCH3C��CCOOCH2CH3����F��A��Ӧ����CH3C��CCOOCH2CH3��CΪCH3CH=CHCHO�����巢���ӳɷ�Ӧ����DΪCH3CHBrCHBrCHO��D��������EΪCH3CHBrCBrCOOH��E������ȥ��Ӧ���ữ�õ�����FΪCH3C��CCOOH���ṹG�Ľṹ��֪��AΪCH3CH2OH��A��������������BΪCH3CHO������Ϣ�з�Ӧ��֪��ȩ�Ц�-H���ã�������ȩ�����ӳɷ�Ӧ����B��Cת����ϵ��֪����ȩ�ȷ����ӳɷ�Ӧ���ٷ���������ȥ��Ӧ����������C��CH3CH=CCHO������1��X����C=C˫���������Ӿ۷�Ӧ���ɸ߷���ճ�ϼ����ṹ��ʽΪ��
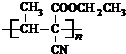 ���ʴ�Ϊ��
���ʴ�Ϊ��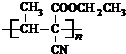 ��
����2��������������֪��CΪCH3CH=CCHO������̼̼˫����ȩ�����ʴ�Ϊ��̼̼˫����ȩ����
��3��A��B��Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2
| Cu |
| �� |
�ʴ�Ϊ��2CH3CH2OH+O2
| Cu |
| �� |
��4������ȩ��Ϊ�Ȼ���̼̼˫���ᱻ������C��D��D��E�ķ�Ӧ˳���ܵߵ���
�ʴ�Ϊ�����ܵߵ���̼̼˫���ᱻ������
��4��C��DΪCH3CH=CCHO���巢���ӳɷ�Ӧ����CH3CHBrCBrCHO��
A+F��G��CH3CH2OH��CH3C��CCOOH����������Ӧ����CH3C��CCOOCH2CH3��
�ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��
��5��CH3C��CCOOH��ͬ���칹���У����������Һ��С�-C��C-���ṹ������CH3COOC��CH��HCOOC��CCH3��У�HC��CCOOCH3��HCOOCH2C��CH��
�ʴ�Ϊ��HC��CCOOCH3��HCOOCH2C��CH��
���������⿼���л��ƶ���ϳɣ��Լ�ϩ��Ȳ����������ȩ�������ת���ȣ��Ƕ��л�������֪ʶ���ۺϿ��飬����ת����ϵ�ķ�Ӧ�������л���ṹ������Ϣ����ʽ������ѧδѧϰ�ķ�Ӧ�Ƚ��������ƶ��ǽ���Ĺؼ����ܽϺõĿ��鿼�����Ķ�����ѧ������˼ά����������ɼ���ϼ�λ���ǽ����������Ŀ�Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

 R-CHO+HCHO
R-CHO+HCHO
 ��
��
 Ϊԭ���Ʊ�
Ϊԭ���Ʊ� ���йػ�ѧ��Ӧ����ʽ�����Լ����ã���
���йػ�ѧ��Ӧ����ʽ�����Լ����ã��� ��2013?��������ģ��2011��Ϊ���ʻ�ѧ�꣬Ҳ��Ϊ�˼��������˷��ַ�����Ԫ���أ�Ra�������ŵ������
��2013?��������ģ��2011��Ϊ���ʻ�ѧ�꣬Ҳ��Ϊ�˼��������˷��ַ�����Ԫ���أ�Ra�������ŵ������