��Ŀ����
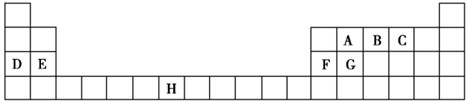
��ͼ��Ԫ�����ڱ���һ���֣����еĢ�~����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش�
|
���� |
��A |
��A |
��A |
��A |
��A |
��A |
��A |
0 |
|
�� |
|
|
|
|
�� |
|
�� |
|
|
�� |
�� |
�� |
�� |
�� |
|
|
�� |
�� |
|
�� |
�� |
|
|
|
|
|
�� |
|
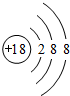
������ЩԪ���У���ѧ��������õ�ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ
 �Ƶؿ��к������Ľ���Ԫ����
�Ƶؿ��к������Ľ���Ԫ����
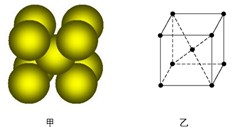
���õ���ʽ��ʾ������γɻ�����Ĺ������������������������������������������߿��ʢ���ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ��������������������ǿ���� �������Ե����������� ��
��д�������������Ʒ�Ӧ�Ļ�ѧ����ʽ����������������������������
��д���ݵ���������������������Һ��Ӧ�����ӷ���ʽ����������������������������
�� ijԪ��R����̬�⻯��ΪHXR����R�ڸ��⻯���е���������Ϊ94%��8.5g��HXR�����ڱ�״̬�µ������5.6L����HXR����Է�����Ϊ��������HXR�Ļ�ѧʽΪ�������� ����
����10�֣�
��1��Ar 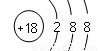
(2)Al
(3)
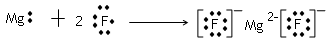
(4)HClO4 KOH Al(OH)3
��5��2Al + 2NaOH + 2H2O =2 NaAlO2+ 3H2��
����Al (OH)3+ OH�� = AlO2��+ 2H2O
��6��34��H2S
��������

 ��
��