ĢāÄæÄŚČŻ
Ļņ50 mL 0.018 mol”¤L-1 AgNO3ČÜŅŗÖŠ¼ÓČė50 mL 0.02 mol”¤L£1ŃĪĖį”£ŅŃÖŖAgCl(s)µÄČܶȻż³£ŹżKsp= 1”Į10£10£¬»ģŗĻŗóČÜŅŗµÄĢå»ż±ä»ÆŗöĀŌ²»¼Ę”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ( )
| A£®»ģŗĻŗó£¬ČÜŅŗÖŠæĻ¶ØÓŠ³ĮµķÉś³É |
| B£®³ĮµķÉś³ÉŗóČÜŅŗÖŠAg+µÄÅضČĪŖ10-5 mol”¤L-1 |
| C£®³ĮµķÉś³ÉŗóČÜŅŗµÄpH£½2 |
| D£®»ģŗĻŗó£¬ÉżøßĪĀ¶Č£¬ČÜŅŗÖŠAg+µÄÅضČŌö“ó |
B
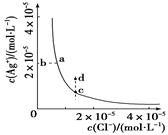
»ģŗĻŗó£¬ČÜŅŗÖŠµÄc (Cl£)="0.01" mol”¤L£1£¬c (Ag£«)="0.009" mol”¤L-1£¬ŌņQc="c" (Cl£)”¤c(Ag£«)=0.01”Į0.009=9”Į10£5£¾1”Į10£10£¬¹ŹŅ»¶ØÓŠ³ĮµķÉś³É£¬AÕżČ·£»Cl£¹żĮ棬ŌņÉś³É³Įµķŗó£¬ČÜŅŗÖŠµÄ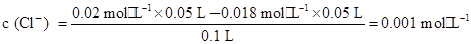 £¬¹Źc(Ag+)=
£¬¹Źc(Ag+)= mol”¤L-1 =10-7 mol”¤L-1£¬B“ķĪó£»»ģŗĻŅŗÖŠµÄc (H£«)="0.01" mol”¤L-1£¬¹ŹpH=2£¬CÕżČ·£»AgClµÄ³ĮµķČܽāĘ½ŗāŹĒĪüČČ·“Ó¦£¬ÉżøßĪĀ¶Č£¬Ę½ŗāĻņČܽāµÄ·½ĻņŅĘ¶Æ£¬ČÜŅŗÖŠAg£«µÄÅضČŌö“ó£¬DÕżČ·”£
mol”¤L-1 =10-7 mol”¤L-1£¬B“ķĪó£»»ģŗĻŅŗÖŠµÄc (H£«)="0.01" mol”¤L-1£¬¹ŹpH=2£¬CÕżČ·£»AgClµÄ³ĮµķČܽāĘ½ŗāŹĒĪüČČ·“Ó¦£¬ÉżøßĪĀ¶Č£¬Ę½ŗāĻņČܽāµÄ·½ĻņŅĘ¶Æ£¬ČÜŅŗÖŠAg£«µÄÅضČŌö“ó£¬DÕżČ·”£
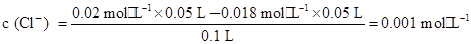 £¬¹Źc(Ag+)=
£¬¹Źc(Ag+)= mol”¤L-1 =10-7 mol”¤L-1£¬B“ķĪó£»»ģŗĻŅŗÖŠµÄc (H£«)="0.01" mol”¤L-1£¬¹ŹpH=2£¬CÕżČ·£»AgClµÄ³ĮµķČܽāĘ½ŗāŹĒĪüČČ·“Ó¦£¬ÉżøßĪĀ¶Č£¬Ę½ŗāĻņČܽāµÄ·½ĻņŅĘ¶Æ£¬ČÜŅŗÖŠAg£«µÄÅضČŌö“ó£¬DÕżČ·”£
mol”¤L-1 =10-7 mol”¤L-1£¬B“ķĪó£»»ģŗĻŅŗÖŠµÄc (H£«)="0.01" mol”¤L-1£¬¹ŹpH=2£¬CÕżČ·£»AgClµÄ³ĮµķČܽāĘ½ŗāŹĒĪüČČ·“Ó¦£¬ÉżøßĪĀ¶Č£¬Ę½ŗāĻņČܽāµÄ·½ĻņŅĘ¶Æ£¬ČÜŅŗÖŠAg£«µÄÅضČŌö“ó£¬DÕżČ·”£
Į·Ļ°²įĻµĮŠ“š°ø
 ½ņĒŽĢÓż¼ĘĖ抔דŌŖĻµĮŠ“š°ø
½ņĒŽĢÓż¼ĘĖ抔דŌŖĻµĮŠ“š°ø
Ļą¹ŲĢāÄæ
 Ag+(aq)+Cl-(aq)
Ag+(aq)+Cl-(aq) 2Na++S2-
2Na++S2-
 )”Ć[c(Cu2+)+c(Fe2+)+c(Fe3+)]>5”Ć4
)”Ć[c(Cu2+)+c(Fe2+)+c(Fe3+)]>5”Ć4 CuS(s)£«Mn2£«(aq)”£ĻĀĮŠÓŠ¹ŲøĆ·“Ó¦µÄĶĘĄķ²»ÕżČ·µÄŹĒ£Ø £©
CuS(s)£«Mn2£«(aq)”£ĻĀĮŠÓŠ¹ŲøĆ·“Ó¦µÄĶĘĄķ²»ÕżČ·µÄŹĒ£Ø £©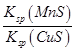
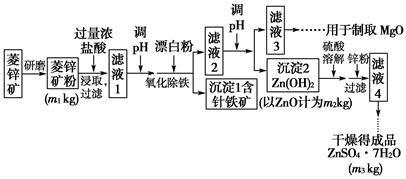
 Ag£«(aq)£«Cl£(aq)ŌŚĖ®ÖŠµÄ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾”£
Ag£«(aq)£«Cl£(aq)ŌŚĖ®ÖŠµÄ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾”£