��Ŀ����
��ҵ��һ���ں����ܱ������в������з�Ӧ�ϳɼ״�:
CO(g) +2H2(g)  CH3OH(g) ��H =-90.8 kJ��mol-1
CH3OH(g) ��H =-90.8 kJ��mol-1
300��ʱ�����ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
���� | �� | �� | �� | |
��Ӧ��Ͷ���� | 1 molCO��2 mol H2 | 1mol CH3OH | 2mol CH3OH | |
ƽ�� ʱ ���� | CH3OH��Ũ��(mol��L-1) | c1 | c2 | c3 |
��Ӧ�������仯 | a kJ | bkJ | ckJ | |
��ϵѹǿ(Pa) | p1 | p2 | p3 | |
��Ӧ��ת���� | a1 | a2 | a3 | |
����˵����ȷ����
A. 2c1>c3 B. �Oa�O+�Ob�O=90.8
C. 2p2<p3 D. a1+ a3<1
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д���֪�������ݣ�
���� | �۵�(��) | �е�(��) | �ܶ�(g��cm��3) |
�Ҵ� | ��117.0 | 78.0 | 0.79 |
���� | 16.6 | 117.9 | 1.05 |
�������� | ��83.6 | 77.5 | 0.90 |
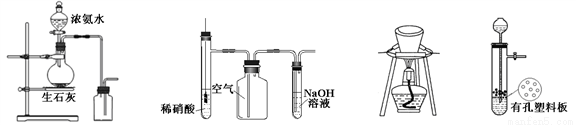
ijѧ����ʵ������ȡ������������Ҫ�������£�

������2 mLŨ���ᡢ3 mL�Ҵ�(��18O)��2 mL����Ļ����Һ��
�ڰ���ͼ���Ӻ�װ��(װ������������)��������Һ����С����ȼ���3 min��5 min��
�۴��Թ����ռ���һ���������ֹͣ���ȣ������Թ��Ҳ�������Ȼ���ô��ֲ㡣
�ܷ��������������ϴ�ӡ����
(1)��Ӧ��Ũ�����������______________________________________��
д����ȡ���������Ļ�ѧ����ʽ��_____________________________��
(2)����ʵ���б���̼������Һ��������________(����ĸ)��
A���к�������Ҵ� B���к����Ტ�����Ҵ�
C�����������������ܽ� D�������������ɣ���������
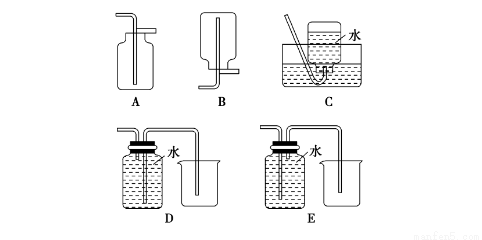
(3)���������ҪС����ȼ��ȣ�����Ҫ������___________________��
��������۲쵽��������_____________________________________��
�������Թ��е����ʷ����Եõ���������������ʹ�õ�������________������ʱ����������Ӧ������________(��¿ڷš����Ͽڵ���)����


 4NO(g)+6H2O(g) ��H=-905.5kJ��mol-1
4NO(g)+6H2O(g) ��H=-905.5kJ��mol-1  ]��ֱ��Ӱ��÷����������ʣ�350��ʱ��ֻ�ı䰱����Ͷ��������Ӧ��X��ת�����백���ȵĹ�ϵ����ͼ��ʾ����X��_______ (��ѧʽ)����������0.5���ӵ�1.0ʱ��������Ҫ��Ӧ��ƽ�⽫��_______�����ƶ�����
]��ֱ��Ӱ��÷����������ʣ�350��ʱ��ֻ�ı䰱����Ͷ��������Ӧ��X��ת�����백���ȵĹ�ϵ����ͼ��ʾ����X��_______ (��ѧʽ)����������0.5���ӵ�1.0ʱ��������Ҫ��Ӧ��ƽ�⽫��_______�����ƶ�����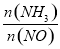 >1.0ʱ��������NOŨ�ȷ���������Ҫԭ����______��
>1.0ʱ��������NOŨ�ȷ���������Ҫԭ����______��
 3Z(g)��b���߱�ʾ��һ��������ѹǿ
3Z(g)��b���߱�ʾ��һ��������ѹǿ