��Ŀ����
���ͷ��ͨ����������ء��������̡�������ʣ�ij�о���ѧϰС����л��ͷ���й����ʵ�ʵ��̽����
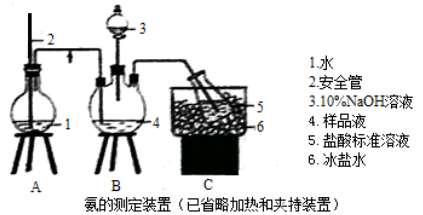
��1��������ͷ�к�����������������ͼ����ʾʵ��װ����©�����棬��һ��ȼ�ŵĻ���ȼ������������ע���������������û��ȼ�ղ���������ͨ��ϡƷ����Һ���۲쵽Ʒ����Һ��ɫ��
��ȼ�ղ�����������һ������______��
�ڿ���������Թ���Ʒ����Һ���Լ���______�����ţ���
A��ϡ�������������Һ B������ʯ��ˮ
C��ϡ��ˮD���ռ���Һ
��ijͬѧ�����ͼ����ʾ��������ʵ���Ϊ��㣮���IJ����ǣ�
i����ͼ������ʾ���ͷȼ����ʱ�������Ƴ����
ii��______��
��2��֤������е���Ԫ����������ص���ʽ���ڣ����������Ȼ��ص���ʽ���ڣ���������в�����
��ȡ3��4�����ժ�»��ͷ������ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У�������������������Һ��û�а�ɫ�������ɣ�
������������Һ�м���ϡ�����NaNO2��Һ���۲쵽�а�ɫ�������ɣ���Ӧ�����ӷ���ʽ��______��
�۽�ȼ���Ļ��ͷ����������ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У��μ�AgNO3��ϡ���ᣬ�ɹ۲쵽��ɫ����������˵����ȼ���Ļ��ͷ����������ˮ�У�����______���ʱ�������˵�����ͷȼ��ʱ���к��е�______ת��Ϊ�����ʣ�
��3���ⶨ���ͷ��KClO3�ĺ�����Ҫʵ�鲽�����£�
��i����ȡ���ͷ��С�����飬�Ƶ�����Ϊ2.45g��
��ii������������ˮ��ֽ��ݺ���ˡ�ϴ�Ӳ�����
��iii����װ����Һ��ϴ��Һ���ձ��м��������NaNO2��Һ��AgNO3��Һ��ϡ���ᣬ���裬��ַ�Ӧ���ˡ�ϴ�ӳ�����
��iv�����������Ƶ�������Ϊ1.435g��
�ٷ�Ӧ��NaNO2��AgNO3����Ҫ������ԭ����______��
��ʵ���û��ͷ��KClO3����������Ϊ______��
�������ii����δϴ�ӳ������������KC1O3������������______���ƫ����ƫС��������Ӱ�족����ͬ��������ڢ���δϴ��AgCl���������KClO3������������______��

��1��������ͷ�к�����������������ͼ����ʾʵ��װ����©�����棬��һ��ȼ�ŵĻ���ȼ������������ע���������������û��ȼ�ղ���������ͨ��ϡƷ����Һ���۲쵽Ʒ����Һ��ɫ��
��ȼ�ղ�����������һ������______��
�ڿ���������Թ���Ʒ����Һ���Լ���______�����ţ���
A��ϡ�������������Һ B������ʯ��ˮ
C��ϡ��ˮD���ռ���Һ
��ijͬѧ�����ͼ����ʾ��������ʵ���Ϊ��㣮���IJ����ǣ�
i����ͼ������ʾ���ͷȼ����ʱ�������Ƴ����
ii��______��
��2��֤������е���Ԫ����������ص���ʽ���ڣ����������Ȼ��ص���ʽ���ڣ���������в�����
��ȡ3��4�����ժ�»��ͷ������ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У�������������������Һ��û�а�ɫ�������ɣ�
������������Һ�м���ϡ�����NaNO2��Һ���۲쵽�а�ɫ�������ɣ���Ӧ�����ӷ���ʽ��______��
�۽�ȼ���Ļ��ͷ����������ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У��μ�AgNO3��ϡ���ᣬ�ɹ۲쵽��ɫ����������˵����ȼ���Ļ��ͷ����������ˮ�У�����______���ʱ�������˵�����ͷȼ��ʱ���к��е�______ת��Ϊ�����ʣ�
��3���ⶨ���ͷ��KClO3�ĺ�����Ҫʵ�鲽�����£�
��i����ȡ���ͷ��С�����飬�Ƶ�����Ϊ2.45g��
��ii������������ˮ��ֽ��ݺ���ˡ�ϴ�Ӳ�����
��iii����װ����Һ��ϴ��Һ���ձ��м��������NaNO2��Һ��AgNO3��Һ��ϡ���ᣬ���裬��ַ�Ӧ���ˡ�ϴ�ӳ�����
��iv�����������Ƶ�������Ϊ1.435g��
�ٷ�Ӧ��NaNO2��AgNO3����Ҫ������ԭ����______��
��ʵ���û��ͷ��KClO3����������Ϊ______��
�������ii����δϴ�ӳ������������KC1O3������������______���ƫ����ƫС��������Ӱ�족����ͬ��������ڢ���δϴ��AgCl���������KClO3������������______��
��1���ٻ��ͷ��ͨ����������ء��������̡���������ȼ�����ɶ����������壬ȼ�ղ�����������һ�����������ʴ�Ϊ����������
�ڼ�������������壬���ö�������Ļ�ԭ�Կ��Ժ���ˮ�����ӳɷ�Ӧʹ��ˮ��ɫ������������������Һ��Ӧ����Һ�Ϻ�ɫ��ȥ������ʯ��ˮ���ռ���Һ���ն�������������
�ʴ�Ϊ��AC��
�۷���װ��ͼ�����ж���Ҫ�IJ��������ͷȼ�����ɶ����������壬����Ѹ�ٽ����ձ��������ձ��ϣ���������ձ����۲�Ʒ����Һ�Ƿ���ɫ�ж����ɶ����������壬��Ʒ����ɫ˵�����ɶ����������壬
�ʴ�Ϊ��Ѹ�ٽ����ձ��������ձ��ϣ���������ձ���
��2���ڼ�����������Һ�������ɣ�˵������KCl������ϡ�����NaNO2��Һ���۲쵽�а�ɫ��������˵������������ӱ��������������Ϊ��������ӣ���������ӱ���ԭΪ�����Ӻ������ӽ�����ɰ�ɫ��������Ӧ�����ӷ���ʽΪ��ClO3-+3NO2-+Ag+=AgCl��+3NO3-��
�ʴ�Ϊ��ClO3-+3NO2-+Ag+=AgCl��+3NO3-��
�۽�ȼ���Ļ��ͷ����������ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У��μ�AgNO3��ϡ���ᣬ�ɹ۲쵽��ɫ����������˵����ȼ���Ļ��ͷ����������ˮ�У��ܳ��Ȼ��أ����������ӣ����������ӵ����ʷ�������ϻ��ͷ�ɷֺ�ȼ�շ����ķ�Ӧ�ж�ȼ�չ������������Ȼ�����˵�����ͷȼ��ʱ���к��е�KClO3ת��ΪKCl��
�ʴ�Ϊ��KCl��KClO3��
��3����NaNO2��AgNO3����Ҫ������Ϊ�˰�����������е���Ԫ��ȫ����ԭΪ�����Ӻ������ӳ�����ʹ�ⶨ�Ľ����ȷ��
�ʴ�Ϊ��ȷ��KClO3����Ԫ��ȫ��ת��ΪAgCl������
��������Ԫ���غ���㣬������ΪAgC���Ƶ�������Ϊ1.435g�����ʵ���=
=0.01mol��KClO3���ʵ���Ϊ0.01mol����ȡ���ͷ��С�����飬�Ƶ�����Ϊ2.45gʵ���û��ͷ��KClO3����������=
��100%=50%��
�ʴ�Ϊ��50%��
��δϴ�ӳ���������Һ�������������ģ��ⶨ���ƫС��δϴ���Ȼ����������ⶨ������������õ���Ԫ�����ʵ������࣬�ⶨ���ƫ��
�ʴ�Ϊ��ƫС��ƫ��
�ڼ�������������壬���ö�������Ļ�ԭ�Կ��Ժ���ˮ�����ӳɷ�Ӧʹ��ˮ��ɫ������������������Һ��Ӧ����Һ�Ϻ�ɫ��ȥ������ʯ��ˮ���ռ���Һ���ն�������������
�ʴ�Ϊ��AC��
�۷���װ��ͼ�����ж���Ҫ�IJ��������ͷȼ�����ɶ����������壬����Ѹ�ٽ����ձ��������ձ��ϣ���������ձ����۲�Ʒ����Һ�Ƿ���ɫ�ж����ɶ����������壬��Ʒ����ɫ˵�����ɶ����������壬
�ʴ�Ϊ��Ѹ�ٽ����ձ��������ձ��ϣ���������ձ���
��2���ڼ�����������Һ�������ɣ�˵������KCl������ϡ�����NaNO2��Һ���۲쵽�а�ɫ��������˵������������ӱ��������������Ϊ��������ӣ���������ӱ���ԭΪ�����Ӻ������ӽ�����ɰ�ɫ��������Ӧ�����ӷ���ʽΪ��ClO3-+3NO2-+Ag+=AgCl��+3NO3-��
�ʴ�Ϊ��ClO3-+3NO2-+Ag+=AgCl��+3NO3-��
�۽�ȼ���Ļ��ͷ����������ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У��μ�AgNO3��ϡ���ᣬ�ɹ۲쵽��ɫ����������˵����ȼ���Ļ��ͷ����������ˮ�У��ܳ��Ȼ��أ����������ӣ����������ӵ����ʷ�������ϻ��ͷ�ɷֺ�ȼ�շ����ķ�Ӧ�ж�ȼ�չ������������Ȼ�����˵�����ͷȼ��ʱ���к��е�KClO3ת��ΪKCl��
�ʴ�Ϊ��KCl��KClO3��
��3����NaNO2��AgNO3����Ҫ������Ϊ�˰�����������е���Ԫ��ȫ����ԭΪ�����Ӻ������ӳ�����ʹ�ⶨ�Ľ����ȷ��
�ʴ�Ϊ��ȷ��KClO3����Ԫ��ȫ��ת��ΪAgCl������
��������Ԫ���غ���㣬������ΪAgC���Ƶ�������Ϊ1.435g�����ʵ���=
| 1.435g |
| 143.5g/mol |
| 0.01mol��122.5g/mol |
| 2.45g |
�ʴ�Ϊ��50%��
��δϴ�ӳ���������Һ�������������ģ��ⶨ���ƫС��δϴ���Ȼ����������ⶨ������������õ���Ԫ�����ʵ������࣬�ⶨ���ƫ��
�ʴ�Ϊ��ƫС��ƫ��

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
�����Ŀ