��Ŀ����
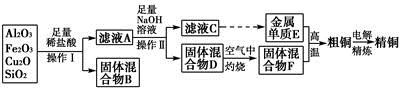
��ҵ����ij����(����Cu2O��Al2O3��Fe2O3��SiO2)��ȡͭ�IJ����������£�
��֪��Cu2O��2H��=Cu��Cu2����H2O
(1)ʵ������������Ϊ________���ڿ��������չ�������Dʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����________(����������)��
(2)��ҺA����Ԫ�صĴ�����ʽΪ________(�����ӷ���)�����ɸ����ӵ����ӷ���ʽΪ____________________________________________��������ҺA�д��ڸ����ӵ��Լ�Ϊ________(���Լ�����)��
(3)��������E���������F������ijһ��Ӧ�����ں��Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________________��
(4)�����£���pH��NaAlO2��NaOH������Һ�У���ˮ�������c(OH��)ǰ��Ϊ���ߵ�108������������Һ��pH��________��
(5)��Ũ���ᡢŨ���ᡢ����ˮ��ѡ�ú��ʵ��Լ����ⶨ��ͭ��Ʒ�н���ͭ�������������漰����Ҫ���裺��ȡһ����������Ʒ��________________�����ˡ�ϴ�ӡ����������ʣ�����ͭ��������(��ȱ�ٵIJ������裬���������������̵�ϸ��)

��֪��Cu2O��2H��=Cu��Cu2����H2O
(1)ʵ������������Ϊ________���ڿ��������չ�������Dʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����________(����������)��
(2)��ҺA����Ԫ�صĴ�����ʽΪ________(�����ӷ���)�����ɸ����ӵ����ӷ���ʽΪ____________________________________________��������ҺA�д��ڸ����ӵ��Լ�Ϊ________(���Լ�����)��
(3)��������E���������F������ijһ��Ӧ�����ں��Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________________��
(4)�����£���pH��NaAlO2��NaOH������Һ�У���ˮ�������c(OH��)ǰ��Ϊ���ߵ�108������������Һ��pH��________��
(5)��Ũ���ᡢŨ���ᡢ����ˮ��ѡ�ú��ʵ��Լ����ⶨ��ͭ��Ʒ�н���ͭ�������������漰����Ҫ���裺��ȡһ����������Ʒ��________________�����ˡ�ϴ�ӡ����������ʣ�����ͭ��������(��ȱ�ٵIJ������裬���������������̵�ϸ��)
(1)���ˡ�����
(2)Fe2����2Fe3����Cu=2Fe2����Cu2�������軯����Һ��������ˮ(����������������)
(3)2Al��Fe2O3 Al2O3��2Fe��(4)11
Al2O3��2Fe��(4)11
(5)��Ũ����������ˮϡ�ͣ����Ƶõ���Ʒ������ϡ�����ַ�Ӧ
(2)Fe2����2Fe3����Cu=2Fe2����Cu2�������軯����Һ��������ˮ(����������������)
(3)2Al��Fe2O3
 Al2O3��2Fe��(4)11
Al2O3��2Fe��(4)11(5)��Ũ����������ˮϡ�ͣ����Ƶõ���Ʒ������ϡ�����ַ�Ӧ
������ͼ���Կ�������������ϡ����ʱ���õ��Ĺ�������BΪ��������͵���ͭ������ҺA��һ������Fe3����ԭ����2Fe3����Cu=2Fe2����Cu2������֤Fe2�����ȼ���KSCN��Һ��û�����������ټ�����ˮ(������������)����Ѫ��ɫ����ҺA�к���Fe2����Al3����Cu2������������NaOH��Һ��õ��Ĺ�������DΪFe(OH)3��Cu(OH)2���ڿ��������պ�õ��Ĺ�������FΪFe2O3�� CuO����ҺC�к�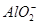 �����������EΪAl���������ȷ�Ӧ�ɺ��Ӹֹ졣(4)NaAlO2��Һ��ˮ�ĵ����ܵ��ٽ�����NaOH��Һ��ˮ�ĵ����ܵ����ơ���������Һ��pH��a��NaAlO2��Һ��ˮ�������c(OH��)Ϊ10a��14mol��L��1��NaOH��Һ��ˮ�������c(OH��)Ϊ10��amol��L��1,10a��14��108��10��a����a��11��(5)��ϡH2SO4�ܽ��Cu�еĻ��ý�����
�����������EΪAl���������ȷ�Ӧ�ɺ��Ӹֹ졣(4)NaAlO2��Һ��ˮ�ĵ����ܵ��ٽ�����NaOH��Һ��ˮ�ĵ����ܵ����ơ���������Һ��pH��a��NaAlO2��Һ��ˮ�������c(OH��)Ϊ10a��14mol��L��1��NaOH��Һ��ˮ�������c(OH��)Ϊ10��amol��L��1,10a��14��108��10��a����a��11��(5)��ϡH2SO4�ܽ��Cu�еĻ��ý�����
 �����������EΪAl���������ȷ�Ӧ�ɺ��Ӹֹ졣(4)NaAlO2��Һ��ˮ�ĵ����ܵ��ٽ�����NaOH��Һ��ˮ�ĵ����ܵ����ơ���������Һ��pH��a��NaAlO2��Һ��ˮ�������c(OH��)Ϊ10a��14mol��L��1��NaOH��Һ��ˮ�������c(OH��)Ϊ10��amol��L��1,10a��14��108��10��a����a��11��(5)��ϡH2SO4�ܽ��Cu�еĻ��ý�����
�����������EΪAl���������ȷ�Ӧ�ɺ��Ӹֹ졣(4)NaAlO2��Һ��ˮ�ĵ����ܵ��ٽ�����NaOH��Һ��ˮ�ĵ����ܵ����ơ���������Һ��pH��a��NaAlO2��Һ��ˮ�������c(OH��)Ϊ10a��14mol��L��1��NaOH��Һ��ˮ�������c(OH��)Ϊ10��amol��L��1,10a��14��108��10��a����a��11��(5)��ϡH2SO4�ܽ��Cu�еĻ��ý�����
��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ

 MgO��H2O
MgO��H2O Mg��Cl2��
Mg��Cl2��