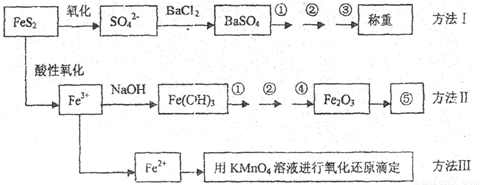
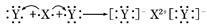��Ŀ����
��֪ij���ʵ���Ҫ�ɷֵĻ�ѧʽΪXY2��Xԭ�ӵĽṹʾ��ͼΪ ��X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ�أ�����ԭ�ӵ�������������������Ӳ�����2����Z��Y������Z��W���γ�һ��WZ2�ͷ��ӣ�
��X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ�أ�����ԭ�ӵ�������������������Ӳ�����2����Z��Y������Z��W���γ�һ��WZ2�ͷ��ӣ�
(1)m��________�������ʵĻ�ѧʽΪ________��
(2)Z��WԪ�ص�����Ϊ__________��__________.
(3)����˵����ȷ����________��
A��XY2��WZ2��Ϊ���ӻ�����
B��XY2�����н������Ӽ���WZ2�н������Թ��ۼ�
C��H2Z��HY���ȶ���ǿ
D��X�������ӱ�Y�������Ӱ뾶��
(4)���л�ѧ���������ȷ����________��
A��XY2�ĵ���ʽ��X2��[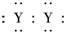 ]2��
]2��
B��WZ2�Ľṹʽ��Z===W===Z
C��YԪ�صĵ�����H2Zˮ��Һ��Ӧ�����ӷ���ʽΪ��Y2��Z2��===2Y����Z��
D���õ���ʽ��ʾXY2���γɹ���Ϊ��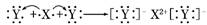
(5)��ѩ�Ļ�ѧ�ɷ���H2O��ˮ�ķе��H2Z�ķе�ߣ���ԭ����_____________________________________________________________________��
(1)20��CaCl2��(2)��̼��(3)B��(4)B��D(5)ˮ���Ӽ�������
�����������ݺ�����ӵ��Ų����ɿ�֪XӦ��CaԪ�أ�X����������Y�������ӵĵ��Ӳ�ṹ��ͬ������Y����Ԫ�ء�ԭ�ӵ�����������������Ӳ�����2���ģ�ֻ��CԪ�غ�SԪ�أ�Z��Y������Z��W���γ�һ��WZ2�ͷ��ӣ�����Z��S��W��C��
��1����2����
��3��CaCl2�����ӻ����ֻ�����Ӽ���CS2�ǹ��ۻ�������м��Լ���A����ȷ��B��ȷ���ȵ��ǽ�����ǿ��S�ģ������Ȼ�����ȶ���ǿ������ģ�C����ȷ�������Ӻ������Ӿ�����ͬ�ĵ��Ӳ�ṹ�������뾶�����ӵĶ�Ӧ�����ӵģ�D����ȷ��
��4��CaCl2�����ӻ����ֻ�����Ӽ�������ʽΪ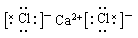 ��CS2��ֱ���ͽṹ��B��ȷ�������������ᣬ���ܸ�д�������γɣ���ȷ�����ӷ���ʽΪCl2��H2S=2H����2Cl����S���������ڻ��ý�����ʧȥ���ӱ��ȵõ�������D��ȷ��
��CS2��ֱ���ͽṹ��B��ȷ�������������ᣬ���ܸ�д�������γɣ���ȷ�����ӷ���ʽΪCl2��H2S=2H����2Cl����S���������ڻ��ý�����ʧȥ���ӱ��ȵõ�������D��ȷ��
��5������ˮ�д��������������е��������ġ�

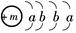 ��X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ�أ�����ԭ�ӵ�������������������Ӳ�����2����Z��Y������Z��W���γ�һ��WZ2�ͷ��ӣ�
��X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ�أ�����ԭ�ӵ�������������������Ӳ�����2����Z��Y������Z��W���γ�һ��WZ2�ͷ��ӣ�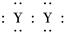 ]2��
]2��