��Ŀ����
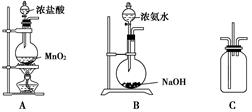
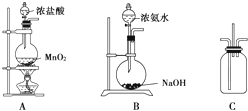
��9�֣�ijУѧ��������ͼ��ʾװ����֤�����백��֮��ķ�Ӧ(����װ������ȥ)������A��B�ֱ�Ϊ�����Ͱ����ķ���װ�ã�CΪ��������������백����Ӧ��װ�á�
��ش��������⣺
��1��װ��A�з�����Ӧ�����ӷ���ʽΪ___________________________________��
��2��װ��B��Ũ��ˮ��NaOH�����Ͽ���ȡ��������ԭ����_____________________��
��3��װ��C�������Ͱ�����������Ũ��İ��̲��������ڱ����ᣬͬʱ����һ�ֳ��������嵥�ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��4����װ��C�з�Ӧ���ɵĹ�������ˮ��������Һ������Ũ���ɴ�С��˳��Ϊ___________.
��1��MnO2��4H����2Cl����Mn2����Cl2����2H2O��2�֣�
��2��NaOH������ˮ�Բ�������ˮʱ�ų������ȣ�OH��Ũ������������ƽ�������ɰ����ķ����ƶ���3�֣�
��3��3Cl2��8NH3===6NH4Cl��N2��2�֣�
��4�� ��2�֣�
��2�֣�
��������
�����������ʵ���Ŀ������֤�����백��֮��ķ�Ӧ��
��1��װ��A����������װ�ã�����������Ũ����������������ķ�Ӧ��ע��Ũ���������ʽΪMnO2��4H����2Cl����Mn2����Cl2����2H2O��
��2���ù����������Ƶ�ԭ�����������ƹ�����ˮ�ų�������ͬʱOH��Ũ������������ƽ�������ɰ����ķ�����������Ҳ���ԴﵽĿ�ġ�
��3����Ũ��İ��̲����Ȼ��⣬���Ȼ�粒��壬���������嵥���ǰ�������ѧ����ʽΪ3Cl2��8NH3===6NH4Cl��N2��
��4���Ȼ����Һ��笠�����Ҫˮ�⣬������Ũ���������Ũ���ɴ�С��˳��Ϊ ��
��
���㣺����ʵ������ȡ�����������������백���ķ�Ӧ������Ũ�ȵıȽϡ�
��������������д������ʽҪע����ȷ�жϲ�����ڼ��⡣

 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�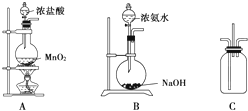 ijУѧ��������ͼ��ʾװ����֤�����백��֮��ķ�Ӧ������װ������ȥ��������A��B�ֱ�Ϊ�����Ͱ����ķ���װ�ã�CΪ��������������백����Ӧ��װ�ã�
ijУѧ��������ͼ��ʾװ����֤�����백��֮��ķ�Ӧ������װ������ȥ��������A��B�ֱ�Ϊ�����Ͱ����ķ���װ�ã�CΪ��������������백����Ӧ��װ�ã�