��Ŀ����
��˫ѡ����1 L�ܱ������з�����Ӧ��4NH3(g)+5O2(g) 4NO(g)+6H2O(g) ��H=��Q kJ��mol��1��Q>O���������ڲ������ʵ����ʵ���Ũ�����±���
4NO(g)+6H2O(g) ��H=��Q kJ��mol��1��Q>O���������ڲ������ʵ����ʵ���Ũ�����±���
����˵���������
A����Ӧ�ڵ�2 min����4minʱ��O2��ƽ����Ӧ����Ϊ0.1875 mol/(L��min)
B����Ӧ�ڵ�2 minʱ�ı���ijһ������������������ʹ�ô����������¶�
C����4 minʱ����8 minʱ�ֱ�ﵽ��ѧƽ�⣬��ƽ�ⳣ������ͬ
D���ڿ�ʼ��Ӧ��ǰ2 min�ڣ��÷�Ӧ�ġ�H=��0.05Q kJ��mol��1
 4NO(g)+6H2O(g) ��H=��Q kJ��mol��1��Q>O���������ڲ������ʵ����ʵ���Ũ�����±���
4NO(g)+6H2O(g) ��H=��Q kJ��mol��1��Q>O���������ڲ������ʵ����ʵ���Ũ�����±���  Ũ�� Ũ��ʱ�� | c(NH3) (mol/L) | c(O2 ) (mol/L) | c(NO) (mol/L) |
| ��ʼ | 0.8 | 1.6 | 0 |
| ��2min | 0.6 | a | 0.2 |
| ��4min | 0.3 | 0.975 | 0.5 |
| ��6min | 0.3 | 0.975 | 0.5 |
| ��8min | 0.7 | 1.475 | 0.1 |
| ��10min | 0.7 | 1.475 | 0.1 |
A����Ӧ�ڵ�2 min����4minʱ��O2��ƽ����Ӧ����Ϊ0.1875 mol/(L��min)
B����Ӧ�ڵ�2 minʱ�ı���ijһ������������������ʹ�ô����������¶�
C����4 minʱ����8 minʱ�ֱ�ﵽ��ѧƽ�⣬��ƽ�ⳣ������ͬ
D���ڿ�ʼ��Ӧ��ǰ2 min�ڣ��÷�Ӧ�ġ�H=��0.05Q kJ��mol��1
BD
������������ɡ�c�¡�t�Ķ���ʽֱ����v(NH3)��v(NO)������ϵ���ȹ��ɼ����v(O2)����v(O2)="0.3mol/L��2min��5/4=0.1875" mol/(L��min)����A��ȷ�������ܼӿ췴Ӧ���ʣ�ʹc(NH3)����������������Ȼ����Ҳ�ܼӿ췴Ӧ���ʣ���Ҳ��ʹƽ�����ƣ�ʹc(NH3)����B����4 minʱ����8 minʱ�ֱ�ﵽ��ѧƽ�⣬����c(H2O)=6c(NO)/4����ƽ�ⳣ���ֱ�Ϊ(0.54��0.756)/(0.34��0.9755)��(0.14��0.156)/(0.74��1.4755)��ǰ�ߴ��ں��ߣ���C��ȷ���ڿ�ʼ��Ӧ��ǰ2 min�ڣ���c(NH3)=0.2mol/L��������0.2mol NH3����ų�������ΪxkJ������4/0.2=Q/x����x=0.05Q����˸÷�Ӧ�ġ�H���ǣ�Q kJ��mol��1����D����

��ϰ��ϵ�д�
�����Ŀ
 H2(g)+I2(g) ��H��0����15s��c(HI)��0.1 mol��L��1����0.07 mol��L��1��������˵����ȷ����
H2(g)+I2(g) ��H��0����15s��c(HI)��0.1 mol��L��1����0.07 mol��L��1��������˵����ȷ���� xC(g)+2D(g)����5min���D��Ũ��Ϊ0.5mol/L��c(A):c(B)=3:5��C��ƽ����Ӧ������0.1 mol/(L��min)����
xC(g)+2D(g)����5min���D��Ũ��Ϊ0.5mol/L��c(A):c(B)=3:5��C��ƽ����Ӧ������0.1 mol/(L��min)����
 CO2(g)��H2(g)
CO2(g)��H2(g)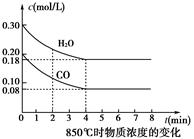
 xC(g)��2D(g)����5min���D��Ũ��Ϊ0.5mol��L��1��c(A)�Uc(B)=3:5��C��ƽ����Ӧ������0.1m0L��(L��min)
xC(g)��2D(g)����5min���D��Ũ��Ϊ0.5mol��L��1��c(A)�Uc(B)=3:5��C��ƽ����Ӧ������0.1m0L��(L��min) 2NH3��������������ͬʱ���ڲ�õĽ���ж�,���ɰ��ķ�Ӧ�ٶ������ǣ�
2NH3��������������ͬʱ���ڲ�õĽ���ж�,���ɰ��ķ�Ӧ�ٶ������ǣ�