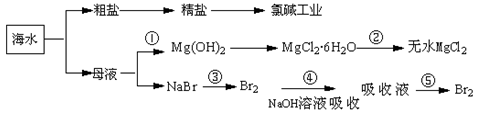
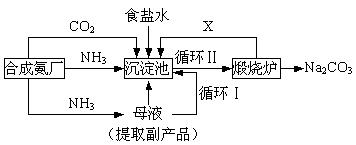
��Ŀ����
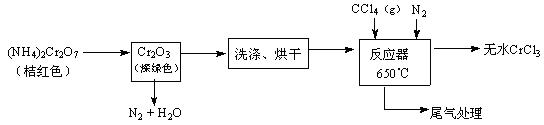
��14�֣����������ô���Ʒ�ӹ����ϣ�������GeO2����Ҫ;��������������ͼ��
��1��Ge2+��������H 2O2��Ӧ����Ge4+��д���÷�Ӧ�����ӷ���ʽ ��
2O2��Ӧ����Ge4+��д���÷�Ӧ�����ӷ���ʽ ��
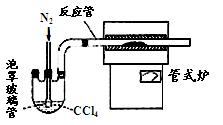
 ��2������ɻ�÷е�ϵ͵�GeCl4���ڴ˹����м���Ũ�����ԭ���� ��ʵ�����������ʱ���õIJ��������У��ƾ��ơ�������ƿ�� �� �����չܡ���ƿ�ȡ�
��2������ɻ�÷е�ϵ͵�GeCl4���ڴ˹����м���Ũ�����ԭ���� ��ʵ�����������ʱ���õIJ��������У��ƾ��ơ�������ƿ�� �� �����չܡ���ƿ�ȡ�
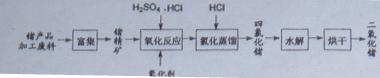
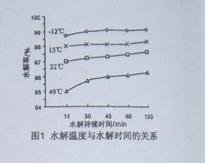
��3��GeCl4ˮ������GeO2��nH2O���˹����û�ѧ����ʽ�ɱ�ʾΪ ���¶ȶ�GeCl4��ˮ���ʲ�����Ӱ����ͼ1��ʾ����ԭ���� ��Ϊ������ѵķ�Ӧ�¶ȡ�ʵ��ʱ�ɲ�ȡ�Ĵ�ʩΪ ��
��
A���ñ�ˮ����� B��49��ˮԡ C���ñ���ˮ
��4�����Ge��Ԫ�����ڱ��е�λ�ü����Խ��ߡ�������GeO2�ܽ�����pH�仯��ԭ�� �������ӷ���ʽ��ʾpH>8ʱGeO2�ܽ���������ܷ����ķ�Ӧ ��

��1��Ge2+��������H
 2O2��Ӧ����Ge4+��д���÷�Ӧ�����ӷ���ʽ ��
2O2��Ӧ����Ge4+��д���÷�Ӧ�����ӷ���ʽ �� ��2������ɻ�÷е�ϵ͵�GeCl4���ڴ˹����м���Ũ�����ԭ���� ��ʵ�����������ʱ���õIJ��������У��ƾ��ơ�������ƿ�� �� �����չܡ���ƿ�ȡ�
��2������ɻ�÷е�ϵ͵�GeCl4���ڴ˹����м���Ũ�����ԭ���� ��ʵ�����������ʱ���õIJ��������У��ƾ��ơ�������ƿ�� �� �����չܡ���ƿ�ȡ���3��GeCl4ˮ������GeO2��nH2O���˹����û�ѧ����ʽ�ɱ�ʾΪ ���¶ȶ�GeCl4��ˮ���ʲ�����Ӱ����ͼ1��ʾ����ԭ���� ��Ϊ������ѵķ�Ӧ�¶ȡ�ʵ��ʱ�ɲ�ȡ�Ĵ�ʩΪ
 ��
��A���ñ�ˮ����� B��49��ˮԡ C���ñ���ˮ

��4�����Ge��Ԫ�����ڱ��е�λ�ü����Խ��ߡ�������GeO2�ܽ�����pH�仯��ԭ�� �������ӷ���ʽ��ʾpH>8ʱGeO2�ܽ���������ܷ����ķ�Ӧ ��


��

��ϰ��ϵ�д�
�����Ŀ


 2Na + Cl2��
2Na + Cl2�� 2Fe + 3CO2��
2Fe + 3CO2��
 4Ag + O2��
4Ag + O2��