��Ŀ����
����ƽ�ⳣ������Ka��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
��1����֪25��ʱ����HF(aq)+OH��(aq)��F��(aq)+H2O(l) ��H����67.7kJ/mol��
��H+(aq)+OH��(aq)��H2O(l) ��H����57.3kJ/mol��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ________________________��
��2����Ũ��Ϊ0.1 mol/LHF��Һ��ˮϡ��һ���������¶Ȳ��䣩�����и����������____��
A��c(H+) B��c(H+)��c(OH��) C��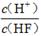 D��
D��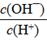
��3��25��ʱ����20mL0.1mol/L������м���VmL0.1mol/LNaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_____��
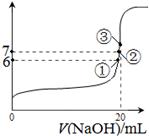
A��pH��3��HF��Һ��pH��11��NaF��Һ�У� ��ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(F��)< c(Na+)��0.1mol/L
��4�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L������������Һ�� �� Na2CO3��Һ �� NaHCO3��Һ�� NaF��Һ ��NaClO��Һ�����������ж�pH�ɴ�С��˳����______________��
��5��Na2CO3��Һ�Լ�������ΪCO32��ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤��֮
___________________________________________________________��
��6������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A��ÿ����1molHFת�� mol���ӡ�
| ��ѧʽ | HF | H2CO3 | HClO |
| ����ƽ�ⳣ�� ��Ka�� | 7.2��10-4 | K1=4.4��10-7 K2=4.7��10-11 | 3.0��10-8 |
��1����֪25��ʱ����HF(aq)+OH��(aq)��F��(aq)+H2O(l) ��H����67.7kJ/mol��
��H+(aq)+OH��(aq)��H2O(l) ��H����57.3kJ/mol��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ________________________��
��2����Ũ��Ϊ0.1 mol/LHF��Һ��ˮϡ��һ���������¶Ȳ��䣩�����и����������____��
A��c(H+) B��c(H+)��c(OH��) C��
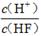 D��
D��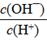
��3��25��ʱ����20mL0.1mol/L������м���VmL0.1mol/LNaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_____��

A��pH��3��HF��Һ��pH��11��NaF��Һ�У� ��ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(F��)< c(Na+)��0.1mol/L
��4�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L������������Һ�� �� Na2CO3��Һ �� NaHCO3��Һ�� NaF��Һ ��NaClO��Һ�����������ж�pH�ɴ�С��˳����______________��
��5��Na2CO3��Һ�Լ�������ΪCO32��ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤��֮
___________________________________________________________��
��6������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A��ÿ����1molHFת�� mol���ӡ�
��1��HF(aq)  H+(aq) +F��(aq) ��H��-10.4KJ��mol��1����д����ſ�1�֣���2��CD
H+(aq) +F��(aq) ��H��-10.4KJ��mol��1����д����ſ�1�֣���2��CD
��3��BC ��4���٢ܢڢۣ����>��>��>��,���������Ʊ�ʾҲ�ԣ���5����̼������Һ�е����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ���dz����6��1
 H+(aq) +F��(aq) ��H��-10.4KJ��mol��1����д����ſ�1�֣���2��CD
H+(aq) +F��(aq) ��H��-10.4KJ��mol��1����д����ſ�1�֣���2��CD ��3��BC ��4���٢ܢڢۣ����>��>��>��,���������Ʊ�ʾҲ�ԣ���5����̼������Һ�е����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ���dz����6��1
���������(1)���ø�˹���ɽ���-�ڿɵ�,HF��aq��?F-��aq��+H+��aq����H="-10.4" kJ?mol-1����2����Ũ��Ϊ0.1 mol/LHF��Һ��ˮϡ��һ����c(H+)��С������ˮ�����ӻ�������c(OH��)����Dѡ����ȷ����3��A���������ˮ�ĵ��룬�δٽ�ˮ�ĵ��룬Aѡ�����B�����ݵ���غ㣬c(Na+)+c(H+)=c(F��)+c(OH��)��ʱ��Һ�У� c(F��)��c(Na+)��c(H+)��c(OH��)=10-6��10-9=9.9��10-7mol/L ��C�����ݵ���غ����Һ�����ԣ����ѵõ�����Һ�е�c(F��)��c(Na+)��D���۵�ʱV��20mL����ʱ�����Һǡ����ȫ��Ӧ���Ƿ���������Һ��������ˮ�⣬��Һ��c(F��)< c(Na+)��0.05mol/L����4����Խ��������Ӧ������Һ����Խǿ�����ԣ�HF��H2CO3��HClO��HCO3- ��������Ӧ�εļ��ԣ�Na2CO3��Һ��NaClO��Һ�� NaHCO3��Һ��NaF��Һ ����5����̼������Һ�е����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ���dz����6��HFO��ԭ������˳��ΪH-O-F�������ɣ�������Ӿ�Ϊ8����H-O�е���ƫ��O��O��-1��O-F����ƫ��F��F�縺�Դ���O����O��+1���ۺ�O�Ļ��ϼ�Ϊ0��F�Ļ��ϼ�-1��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
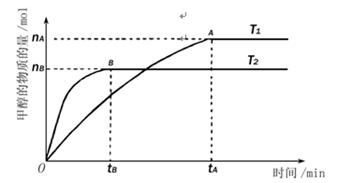
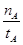 (mol��L-1��min-1)
(mol��L-1��min-1)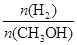 ��С
��С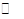 H2O(l)+H2NCONH2(l) ��H=-103��7 kJ��mol-1
H2O(l)+H2NCONH2(l) ��H=-103��7 kJ��mol-1
 2NH3 (g) ��H =" -92.4" kJ/mol
2NH3 (g) ��H =" -92.4" kJ/mol
 2NH3 (g)��ü�������H2��ת����Ϊ40%��
2NH3 (g)��ü�������H2��ת����Ϊ40%�� SO3(g) +NO(g)��
SO3(g) +NO(g)��


 ��
�� ����ԭ
����ԭ �ķ���Ҳ�������������������Ⱦ�����磺
�ķ���Ҳ�������������������Ⱦ�����磺 CH3OH(g)
CH3OH(g)


