��Ŀ����
��1����֪2H2��g��+O2��g��=2H2O��l����H����571.6 kJ/mol��
CO��g����1/2O2��g����CO2��g����H����283.0 kJ/mol��ijH2��CO�Ļ��������ȫȼ��ʱ�ų�113.74 kJ������ͬʱ����3.6 gҺ̬ˮ����ԭ���������H2��CO�����ʵ���֮��Ϊ___________��
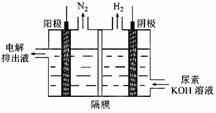
��2���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫���ɹ���ȼ�ϵ�أ���֪��ȼ�ϵ�ص��ܷ�Ӧʽ�ǣ�2CH3OH +3O2+4OH-=2CO32-+6H2O����ȼ�ϵ�ط�����Ӧʱ����������Һ��PH__________ (����� ����С�� ���䡱)�õ�صĸ�����ӦʽΪ_________________��
��3�� ������ȼ�ϵ�ؽ��д�ͭ�ľ�������ͭӦ���ӵ�Դ��________�����ô�ͭ�������ص�������ӦʽΪ_________________��
CO��g����1/2O2��g����CO2��g����H����283.0 kJ/mol��ijH2��CO�Ļ��������ȫȼ��ʱ�ų�113.74 kJ������ͬʱ����3.6 gҺ̬ˮ����ԭ���������H2��CO�����ʵ���֮��Ϊ___________��
��2���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫���ɹ���ȼ�ϵ�أ���֪��ȼ�ϵ�ص��ܷ�Ӧʽ�ǣ�2CH3OH +3O2+4OH-=2CO32-+6H2O����ȼ�ϵ�ط�����Ӧʱ����������Һ��PH__________ (����� ����С�� ���䡱)�õ�صĸ�����ӦʽΪ_________________��
��3�� ������ȼ�ϵ�ؽ��д�ͭ�ľ�������ͭӦ���ӵ�Դ��________�����ô�ͭ�������ص�������ӦʽΪ_________________��
��1��1��1......2��
��2������.1�� CH3OH -6e����8OH-��1����CO32-��6H2O ....2��
��3������..1�֡� Cu2+ + 2e- =Cu��.2��
��2������.1�� CH3OH -6e����8OH-��1����CO32-��6H2O ....2��
��3������..1�֡� Cu2+ + 2e- =Cu��.2��
����������⣺ˮ�����ʵ���Ϊ=0.2mol����2H2+O2�T2H2O��֪��n��H2��=n��H2O��=0.2mol����2H2��g��+O2��g���T2H2O��l����H=-571.6kJ?mol-1��֪��0.2molH2ȼ�շų�������Ϊ57.16KJ����COȼ�շų�������Ϊ113.74KJ-57.16KJ=56.58KJ������������CO�����ʵ���Ϊx����
CO��g��+
 O2��g��=CO2��g����H=-283kJ?mol-1
O2��g��=CO2��g����H=-283kJ?mol-11 283KJ
X 56.58KJ
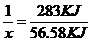
���x=0.2mol����n��CO��=0.20mol����ԭ���������H2��CO�����ʵ���֮��Ϊ1:1.��2���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�أ�������ӦΪ��3O2+12H2O+12e-=12OH-���ܷ�ӦʽΪ��2CH3OH+3O2+4OH-=2CO32-+6H2O����ʽ�����������ӦΪ��2CH3OH-12e-+16OH-=2CO32-+12H2O����3���͵�Դ�����������ĵ缫����������ⷽ��������ͭ�����ص����������Ǵ�ͭ���缫��ӦΪ��Cu-2e-=Cu2+

��ϰ��ϵ�д�
�����Ŀ
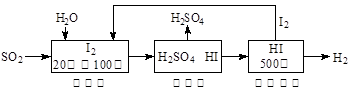
 Ni(OH)2��M
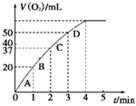
Ni(OH)2��M N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
 N2��2CO2 ��H<0�о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʣ�Ϊ�˷ֱ���֤�¶ȡ������ıȱ�����Ի�ѧ�� Ӧ���ʵ�Ӱ����ɡ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ������±��С�
N2��2CO2 ��H<0�о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʣ�Ϊ�˷ֱ���֤�¶ȡ������ıȱ�����Ի�ѧ�� Ӧ���ʵ�Ӱ����ɡ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ������±��С�

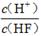 D��
D��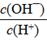
 X��g������H����dJ��mol��1��d��0��XΪA��B��C����Ԫ����ɵ�һ�ֻ��������ʼͶ����������ﵽƽ��ʱ���й��������£�
X��g������H����dJ��mol��1��d��0��XΪA��B��C����Ԫ����ɵ�һ�ֻ��������ʼͶ����������ﵽƽ��ʱ���й��������£�