��Ŀ����
��Ȫ��һ�ֳ�������Ȼ���������ԭ���Ǵ���ѹǿ�
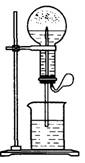
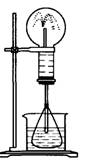
ͼ1 ͼ2
��1��ͼ1Ϊ��ѧ��ѧ�����õ���Ȫʵ��װ�á�����ƿ�г����������壬��ͷ�ιܼ��ձ��зֱ�ʢ��Һ�塣��������в������γ���Ȫ����____
A. HCl��H2O B. O2��H2O C. NH3��H2O D. CO2��NaOH
��2��ijѧ������˼��������Ȫ�������취�����������ͼ2��ʾ��װ�á�
����ͼ2����ƿ�ڣ��ֱ���������������ʣ���Ӧ����ܲ�����Ȫ����__________
A. Cu��ϡ���� B. NaHCO3��NaOH
C. CaCO3��ϡ���� D. NH4HCO3��ϡ����
����ͼ2����ƿ���һˮ�ۣ���ƿ�м���ƾ���ˮ���м����ˮ���ټ����������������ʣ����Ҳ��������Ȫ��ˮ���м�������ʿ�����_______
A. Ũ���� B. ʳ�� C. ����� D. ����ͭ
�۱Ƚ�ͼ1��ͼ2����װ�ã��Ӳ�����Ȫ��ԭ����������ͼ1��_______�ϲ���ƿ������ѹǿ��ͼ2��_______�²���ƿ������ѹǿ���������С����
��3�������г�����������Ȫ����ɽ�緢��ԭ��������__________���ͼ1����ͼ2����װ�õ�ԭ�����ơ�
��12��,ÿ��2�֣���1��B ��2����D ��A �ۼ�С������ ��3��ͼ2

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�| A��������ˮ | B��������̼������������Ʊ���Һ | C���Ȼ��������ˮ | D��һ������������������Ʊ���Һ |
 ��Ȫ��һ�ֳ�������Ȼ����ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�ã���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ʾ��װ�ã���ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ���ǣ�������
��Ȫ��һ�ֳ�������Ȼ����ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�ã���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ʾ��װ�ã���ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ���ǣ������� ��Ȫ��һ�ֳ�������Ȼ�����������ԭ���Ǵ���ѹǿ���ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����
��Ȫ��һ�ֳ�������Ȼ�����������ԭ���Ǵ���ѹǿ���ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����

