��Ŀ����
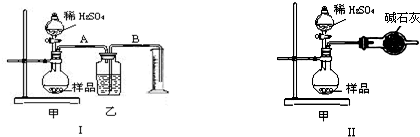
ijУ��ѧ��ȤС���ͬѧ�õζ�����һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ��ʵ�鲽�����£��ش��������⣺
��1���÷�����ƽȷ��ȡ����Ʒ5.000g��ȫ������ˮ���Ƴ�1000.0mL����Һ����
��2����Ũ��Ϊ0.1000mol/L���������Һ���еζ����ζ�����ʹ��ǰӦ�ȼ�©��ϴ�ӡ�
��3�����òⶨ��ҺpH�ķ�����ȷ���ζ����յ㣬��Ӧ��
��4���ζ������У���ƿ����Һ��pH�仯����
����Ƴ������к͵ζ������ߣ�
��5���±��Ǽ������ָʾ���ı�ɫ��Χ���������������к͵ζ����߷����������к͵ζ��п�ѡ�õ�ָʾ����
��6����Ʒ�У�NaOH�������ٷֺ���Ϊ
��1���÷�����ƽȷ��ȡ����Ʒ5.000g��ȫ������ˮ���Ƴ�1000.0mL����Һ����
��ʽ�ζ���
��ʽ�ζ���
��ȡ����20.00mL������ƿ�У��μӼ���ָʾ�������⣮��2����Ũ��Ϊ0.1000mol/L���������Һ���еζ����ζ�����ʹ��ǰӦ�ȼ�©��ϴ�ӡ�
�ô�װҺ��ϴ
�ô�װҺ��ϴ
����ʽװҺ���ų����ݲ�����Һ�森��3�����òⶨ��ҺpH�ķ�����ȷ���ζ����յ㣬��Ӧ��
pH��
pH��
�ⶨ��ƿ����Һ��pH���ٽ��ζ��յ�ʱӦע��ÿ��һ�β�һ��
ÿ��һ�β�һ��
����4���ζ������У���ƿ����Һ��pH�仯����
| V��HCl��/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
| pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
��5���±��Ǽ������ָʾ���ı�ɫ��Χ���������������к͵ζ����߷����������к͵ζ��п�ѡ�õ�ָʾ����
���Ȼ��̪
���Ȼ��̪
���ζ��յ����������Һ�ɻ�ɫ��ɳ�ɫ�����Ӳ���ɫ������Һ�ɷۺ�ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ��
��Һ�ɻ�ɫ��ɳ�ɫ�����Ӳ���ɫ������Һ�ɷۺ�ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ��
�������м��֣�������һ�֣�| ָʾ�� | ��ɫ��Χ ��pH�� |
����Χ����ɫ | ||
| ǰ | �м� | �� | ||
| ���� | 3.1��4.4 | �� | ��ɫ | �� |
| ʯ�� | 5.0��8.0 | �� | ��ɫ | �� |
| ��̪ | 8.2��10.0 | �� | �ۺ� | �� |
96%
96%
�����ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ�����û�����ݣ������ɼ���ֵƫ��
ƫ��
���ƫ����ƫС������ȷ����������������1������������Һ����ǿ�Ӧ��ʹ�ü�ʽ�ζ��ܣ�
��2�����ݵζ��ܵ�ʹ�÷�����ɣ�
��3����Ҫȷ������Һ��pH�仯��Ӧ��ʹ��pH�ƣ��ٽ��ζ��յ�ʱӦע���β�����Һ��pH��
��4������ͼ�еĵζ�������ɵζ������ߣ�
��5������ͼ���ͼ�����ݷ�����PHͻ�䣬����pHͻ��ѡ��ָʾ�������ݵζ�����ǰ��Һ��ʾ���ԣ��ζ�����ʱ�������ᣬ��Һ�������Խ����жϵζ��յ㣻
��6���������ĵı�Һ��������������������Һ��Ũ�ȣ��ټ������Ʒ�к��е��������Ƶ������������������ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ�����û�����ݣ����µζ�ʱ���ĵı�Һ���ƫ��
��2�����ݵζ��ܵ�ʹ�÷�����ɣ�
��3����Ҫȷ������Һ��pH�仯��Ӧ��ʹ��pH�ƣ��ٽ��ζ��յ�ʱӦע���β�����Һ��pH��
��4������ͼ�еĵζ�������ɵζ������ߣ�
��5������ͼ���ͼ�����ݷ�����PHͻ�䣬����pHͻ��ѡ��ָʾ�������ݵζ�����ǰ��Һ��ʾ���ԣ��ζ�����ʱ�������ᣬ��Һ�������Խ����жϵζ��յ㣻
��6���������ĵı�Һ��������������������Һ��Ũ�ȣ��ټ������Ʒ�к��е��������Ƶ������������������ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ�����û�����ݣ����µζ�ʱ���ĵı�Һ���ƫ��
����⣺��1���ζ�ʱ������������Һʹ�ü�ʽ�ζ���ʢȡ���ʴ�Ϊ����ʽ�ζ��ܣ�
��2���ζ�����ʹ��ǰӦ�ȼ�©��ϴ�ӡ��ô�װҺ��ϴ����ʽװҺ���ų����ݲ�����Һ�棬
�ʴ�Ϊ���ô�װҺ��ϴ��
��3�����òⶨ��ҺpH�ķ�����ȷ���ζ����յ㣬pH��������������ҺpH���������ܽϷ���ȷ�زⶨ��Һ��pH���ڵζ��յ㸽����Һ��pH�����ͻ�䣬���Դ�ʱ��ÿ��һ�β�һ��
�ʴ�Ϊ��pH�ƣ�ÿ��һ�β�һ�Σ�
��4�����ݱ������ݿ��Ի��Ƴ��к͵ζ�������Ϊ��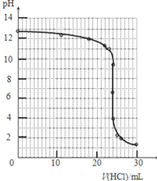 ��
��
�ʴ�Ϊ�� ��
��
��5������ͼ���ͼ�����ݷ�����֪pHͻ����4.0��9.9���������������ǡ�÷�Ӧ���ɵ��Ȼ�����Һ�����ԣ����ȱ�ɫ��ΧΪ3.1-4.4����̪pH��ɫ��ΧΪ8.2��10.0�����Ⱥͷ�̪����������ָʾ��������ʯ���ɫʱ�������жϣ�һ�㲻ѡ��ָʾ����
�ζ�����ǰ����ҺΪ���ԣ���Һ��ɫΪ��ɫ���ζ�����ʱ�зۺ�ɫ�����ɫ�����Եζ��յ����������Һ�ɻ�ɫ��ɳ�ɫ�����Ӳ���ɫ������Һ�ɷۺ�ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ����
�ʴ�Ϊ�����Ȼ��̪����Һ�ɻ�ɫ��ɳ�ɫ�����Ӳ���ɫ������Һ�ɷۺ�ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ����
��6������������Һ��Ũ��Ϊ��
=0.1200mol/L��
��Ʒ�к��е��������Ƶ����ʵ���Ϊ��0.1200mol/L��1L=0.12mol���������Ƶ�����Ϊ��40g/mol��0.12mol=4.800g��
����Ʒ�У�NaOH�������ٷֺ���Ϊ��
��100%=96%��
�ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ�����û�����ݣ��������ĵı�Һ���ƫ�ⶨ���ƫ��
��Ϊ��96%��ƫ��
��2���ζ�����ʹ��ǰӦ�ȼ�©��ϴ�ӡ��ô�װҺ��ϴ����ʽװҺ���ų����ݲ�����Һ�棬
�ʴ�Ϊ���ô�װҺ��ϴ��
��3�����òⶨ��ҺpH�ķ�����ȷ���ζ����յ㣬pH��������������ҺpH���������ܽϷ���ȷ�زⶨ��Һ��pH���ڵζ��յ㸽����Һ��pH�����ͻ�䣬���Դ�ʱ��ÿ��һ�β�һ��
�ʴ�Ϊ��pH�ƣ�ÿ��һ�β�һ�Σ�
��4�����ݱ������ݿ��Ի��Ƴ��к͵ζ�������Ϊ��
 ��
���ʴ�Ϊ��
 ��
����5������ͼ���ͼ�����ݷ�����֪pHͻ����4.0��9.9���������������ǡ�÷�Ӧ���ɵ��Ȼ�����Һ�����ԣ����ȱ�ɫ��ΧΪ3.1-4.4����̪pH��ɫ��ΧΪ8.2��10.0�����Ⱥͷ�̪����������ָʾ��������ʯ���ɫʱ�������жϣ�һ�㲻ѡ��ָʾ����
�ζ�����ǰ����ҺΪ���ԣ���Һ��ɫΪ��ɫ���ζ�����ʱ�зۺ�ɫ�����ɫ�����Եζ��յ����������Һ�ɻ�ɫ��ɳ�ɫ�����Ӳ���ɫ������Һ�ɷۺ�ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ����
�ʴ�Ϊ�����Ȼ��̪����Һ�ɻ�ɫ��ɳ�ɫ�����Ӳ���ɫ������Һ�ɷۺ�ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ����
��6������������Һ��Ũ��Ϊ��
| 0.1000mol/L��0.024L |
| 0.02L |
��Ʒ�к��е��������Ƶ����ʵ���Ϊ��0.1200mol/L��1L=0.12mol���������Ƶ�����Ϊ��40g/mol��0.12mol=4.800g��
����Ʒ�У�NaOH�������ٷֺ���Ϊ��
| 4.800g |
| 5.000g |
�ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ�����û�����ݣ��������ĵı�Һ���ƫ�ⶨ���ƫ��
��Ϊ��96%��ƫ��
���������⿼��������к͵ζ�ʵ��IJ������衢��Ӧ�յ��ͼ����ơ��յ��жϣ���Ŀ�Ѷ��еȣ�ָʾ����ѡ���ǽ���ؼ���

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ