��Ŀ����
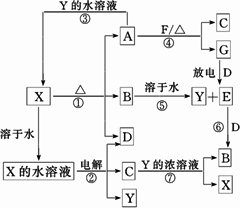
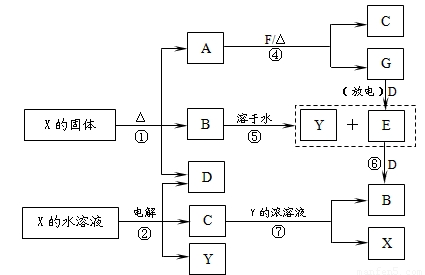
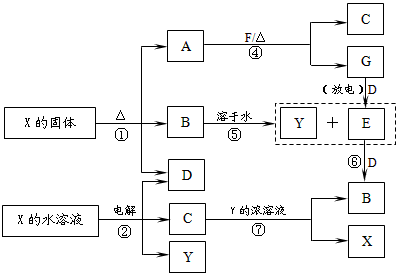
A��G��X��Y������ѧ��ѧ�������ʣ�����B��D��E��F��G�ڳ�����Ϊ������BΪ����ɫ��C�Ǻ�ɫ�Ľ������ʡ�����֮��������ת����ϵ�����з�Ӧ�ۢܢߵIJ����е�ˮ����ȥ����
�����
��1��д��G���ӵĵ���ʽ____________��
��2��д����Ӧ�ߵ����ӷ���ʽ_____________________��
��3��д�����X��Һ��������Ӧʽ______________________��
��4��д����Ӧ�ܵĻ�ѧ����ʽ______________________��
��5����ʹF������;����ȫת��ΪY��
��1��д��G���ӵĵ���ʽ____________��
��2��д����Ӧ�ߵ����ӷ���ʽ_____________________��
��3��д�����X��Һ��������Ӧʽ______________________��
��4��д����Ӧ�ܵĻ�ѧ����ʽ______________________��
��5����ʹF������;����ȫת��ΪY��
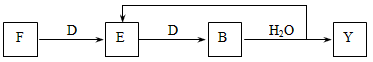
��μӷ�Ӧ��F����������������D�����ʵ���֮��Ϊ��___________��
��1��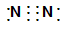
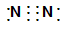
��2��Cu+4H++2NO3-��Cu2++2NO2��+2H2O
��3��4OH--4e-��O2��+2H2O ����2H2O-4e- ��O2��+2H+ ��
��4��2NH3+3CuO N2+3Cu+3H2O
N2+3Cu+3H2O
��5��1:2
��3��4OH--4e-��O2��+2H2O ����2H2O-4e- ��O2��+2H+ ��
��4��2NH3+3CuO
 N2+3Cu+3H2O
N2+3Cu+3H2O ��5��1:2

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ