��Ŀ����
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ����������SO2��ijͬѧ�������ʵ����ȷ��������������к�����ϩ��SO2��
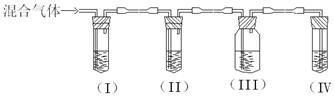
I��II��III��IVװ�ÿ�ʢ�ŵ��Լ��ǣ�I�� II�� III��
IV�� ���뽫�����й��Լ����������ո��ڣ�
��2����˵��SO2������ڵ������� ��
ʹ��װ��II��Ŀ���� ��
ʹ��װ��III��Ŀ���� ��
ȷ��������ϩ�������� ��

I��II��III��IVװ�ÿ�ʢ�ŵ��Լ��ǣ�I�� II�� III��
IV�� ���뽫�����й��Լ����������ո��ڣ�
| A��Ʒ�� | B��NaOH��Һ | C��Ũ���� | D������KMnO4��Һ |
ʹ��װ��II��Ŀ���� ��
ʹ��װ��III��Ŀ���� ��
ȷ��������ϩ�������� ��
ÿ��1�֣���8��
(1) I�� A II�� B III�� A IV�� D
(2) I��Ʒ����Һ��ɫ �� ����SO2 �� ֤��SO2�Ѿ�����ȫ���� ��
IV����Һ��ɫ��dz����ɫ ��
(1) I�� A II�� B III�� A IV�� D
(2) I��Ʒ����Һ��ɫ �� ����SO2 �� ֤��SO2�Ѿ�����ȫ���� ��
IV����Һ��ɫ��dz����ɫ ��
�����������1���������������Ʒ����Һ��������ϩ�ø������������Һ����ϩ�Ͷ���������ʹ�������������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ����SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫ������ϩ����װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ��II�Թ�װ��NaOH��Һ��ȥSO2��װ��III�Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ��IV ͨ���������������Һ��ɫ������ϩ��
�ʴ�Ϊ��A��B��A��D��
��2��װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2���ʴ�Ϊ������Ʒ����Һ��ɫ��
װ�â��Թ�װ��NaOH��Һ��ȥSO2���ʴ�Ϊ������SO2��
װ�â��Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ����ʴ�Ϊ������SO2�Ƿ��Ѿ�����ȫ���գ�
װ�â�ͨ���������������Һ��ɫ������ϩ���ʴ�Ϊ�����е�Ʒ�첻��ɫ�����еĸ��������Һ��ɫ��
���������⿼������ϩ��ʵ�����Ʒ��Լ�����ļ��飬ע��ж��ֲ��������ʱ��Ӧ�����Ⱥ�˳������ؼ�������������������������ϩ���ǽ����Ĺؼ���

��ϰ��ϵ�д�
 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ




