��Ŀ����
��5�֣�����һƿŨ��Ϊ0.2 mol/L��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֡�Ϊ��ȷ��������Һ����ɽ���ʵ�飺ȡ25.00 mL0.1 mol/L������������Һ����μ��������Һ��ǡ�÷�Ӧ��ȫʱ���������Һ���Ϊ12.50 mL����ش�
��1����������� ��
��2����pH��ֽ��÷�Ӧ��������Һ�ʼ��ԣ����ݴ�����˵��������ҺΪ �������ӷ���ʽ˵����Һ�ʼ��Ե�ԭ�� ��
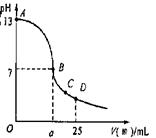
��3��ʵ���еζ���������ͼ����B�㣬a 12.5������ڡ�С�ڻ���ڣ���C�������Ũ���ɴ�С���� ��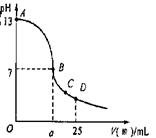
��1�����ᣨ1�֣�
��2�����ᣬCH3COO_ + H2O  CH3COOH + OH_ ����1�֣�
CH3COOH + OH_ ����1�֣�
��3������ �� c��CH3COO���� > c��Na +�� > c��H+�� > c��OH������2�֣�
����

��ϰ��ϵ�д�
�����Ŀ