��Ŀ����
�ֱ�ȡ40mL��0.50mol/L������40mL��0.55mol/L����������Һ�����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ���ش��������⣮
��1��������ϡǿ�ᡢϡǿ�Ӧ����1molˮʱ�ų�57.3kJ��������д����ʾϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ______��
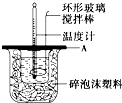
��2����ͼ��ʾ��AΪ��ĭ���ϰ壬����������С�ף��ֱ�����¶ȼƺͻ��β��������������С�ײ��ܿ��ù�����ԭ����______��
��3���������������������Һ���ܶȶ���1g/cm3����֪�кͺ�������Һ�ı�����c=4.18J/��g?�棩��Ϊ�˼����к��ȣ�ʵ��ʱ��������������У�����ţ�______��
A����Ӧǰ������Һ���¶�
B����Ӧǰ������Һ������
C����Ӧǰ����������Һ���¶�
D����Ӧǰ����������Һ������
E����Ӧ������Һ������¶�
F����Ӧ������Һ������
��4��ijѧ��ʵ���¼�������£�
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к��ȡ�H=______��
��1��������ϡǿ�ᡢϡǿ�Ӧ����1molˮʱ�ų�57.3kJ��������д����ʾϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ______��
��2����ͼ��ʾ��AΪ��ĭ���ϰ壬����������С�ף��ֱ�����¶ȼƺͻ��β��������������С�ײ��ܿ��ù�����ԭ����______��
��3���������������������Һ���ܶȶ���1g/cm3����֪�кͺ�������Һ�ı�����c=4.18J/��g?�棩��Ϊ�˼����к��ȣ�ʵ��ʱ��������������У�����ţ�______��
A����Ӧǰ������Һ���¶�
B����Ӧǰ������Һ������
C����Ӧǰ����������Һ���¶�
D����Ӧǰ����������Һ������
E����Ӧ������Һ������¶�
F����Ӧ������Һ������
��4��ijѧ��ʵ���¼�������£�
| ʵ�� ��� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | �������� | �����Һ | |
| 1 | 20.0 | 20.1 | 23.2 |
| 2 | 20.2 | 20.4 | 23.4 |
| 3 | 20.5 | 20.6 | 23.6 |

��1����֪ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������ϡ�������������ϡ��Һ����ǿ���ǿ���ϡ��Һ����Ӧ���Ȼ�ѧ����ʽΪ��NaOH��aq��+
H2SO4��aq���T
Na2SO4��aq��+H2O ��l����H=-57.3 kJ/mol��
�ʴ�Ϊ��NaOH��aq��+
H2SO4��aq���T
Na2SO4��aq��+H2O��l����H=-57.3 kJ/mol��
��2����ͼ��ʾ��AΪ��ĭ���ϰ壬����������С�ף��ֱ�����¶ȼƺͻ��β����������������С���ù��ᵼ��ɢʧ�϶��������Ӱ��ⶨ�����
�ʴ�Ϊ����������ɢʧ��
��3����Q=cm��T��֪���ⶨ�к�����Ҫ�ⶨ������Ϊ��A����Ӧǰ������Һ���¶ȡ�B����Ӧǰ������Һ��������E����Ӧ������Һ������¶ȣ�
��ѡACE��
��4����1��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.05�棬��Ӧ���¶�Ϊ��23.2�棬��Ӧǰ���¶Ȳ�Ϊ��3.15�棻
��2��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.3�棬��Ӧǰ���¶Ȳ�Ϊ��3.1�棻
��3��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.55�棬��Ӧǰ���¶Ȳ�Ϊ��3.05�棻
40mL��0.50mol/L������40mL��0.55mol/L����������Һ��������Ϊm=80mL��1g/cm3=80g��c=4.18J/��g?�棩�����빫ʽQ=cm��T������0.05mol��ˮ�ų�����Q=4.18J/��g?�棩��80g��
=1.036kJ��������0.02mol��ˮ�ų�����Ϊ��1.036kJ����������1mol��ˮ�ų�����Ϊ��1.036kJ��
=-51.8kJ/mol������ʵ���õ��к��ȡ�H=-51.8kJ/mol��
�ʴ�Ϊ��-51.8kJ/mol��
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��NaOH��aq��+
| 1 |
| 2 |
| 1 |
| 2 |
��2����ͼ��ʾ��AΪ��ĭ���ϰ壬����������С�ף��ֱ�����¶ȼƺͻ��β����������������С���ù��ᵼ��ɢʧ�϶��������Ӱ��ⶨ�����
�ʴ�Ϊ����������ɢʧ��
��3����Q=cm��T��֪���ⶨ�к�����Ҫ�ⶨ������Ϊ��A����Ӧǰ������Һ���¶ȡ�B����Ӧǰ������Һ��������E����Ӧ������Һ������¶ȣ�
��ѡACE��
��4����1��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.05�棬��Ӧ���¶�Ϊ��23.2�棬��Ӧǰ���¶Ȳ�Ϊ��3.15�棻
��2��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.3�棬��Ӧǰ���¶Ȳ�Ϊ��3.1�棻
��3��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.55�棬��Ӧǰ���¶Ȳ�Ϊ��3.05�棻
40mL��0.50mol/L������40mL��0.55mol/L����������Һ��������Ϊm=80mL��1g/cm3=80g��c=4.18J/��g?�棩�����빫ʽQ=cm��T������0.05mol��ˮ�ų�����Q=4.18J/��g?�棩��80g��
| 3.15��+3.1��+3.05 |
| 3 |
| 1mol |
| 0.02mol |
�ʴ�Ϊ��-51.8kJ/mol��

��ϰ��ϵ�д�
�����Ŀ