��Ŀ����
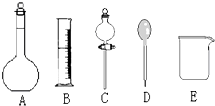 ��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�250mL 0.1mol?L-1��������Һ��
��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�250mL 0.1mol?L-1��������Һ����1������ͼ��ʾ�����У�����������Һ����Ҫ����
C
C
������ͼ��Ӧ��������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������������
������
�������ð�ʹ�õ��Ⱥ�˳��ֱ�������
����
������
����
����2�����ݼ��㣬�������̻����У���ʵ����ͲӦʹ�õ���
A
A
������ƿӦʹ��C
C
�����ں�������д��Ӧ����ţ������֣���ͬ��A��10mL B��100mL C��250mL D��500mL
��3��������ƿ��ʹ�÷����У����в�����ȷ����
AE
AE
��A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ��ˮϴ����������õ�ϡHCl��Һ��ϴ
C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶���
D��������Һʱ����������Һ�壬����Ͳȡ������ֱ�ӵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶���
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ��
��4�������ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ���ʱӦ��
A
A
A��ֱ��ת�Ƶ�ϸ���Լ�ƿ�� B�����ý�ͷ�ιܼ�ˮ�����¶���
C��������Һ�������������� D��ֱ������������ƿ��
��5����������ʱ������������ȷ��ֻ��������ijһ��������ж������Ƶ���ҺŨ�������Ҫ���ֵ����0.1mol/L����Σ���a��ƫ�ߣ�b��ƫ�ͣ�c����Ӱ�죬�����к���������Ӧ��ţ�
������ƿ������������ˮ
c
c
��ϡ��ŨHClʱ��û����ȴ������ת�Ƶ�����ƿ��
a
a
�����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ��
b
b
��������ʱ���ӣ�������Һ�����ʵ���Ũ��
a
a
����������1����������һ�����ʵ���Ũ�ȵ���Һʹ�õ�����������Ҫ��������ȱ�ٵ��������ٸ��ݲ������ڲ����е����ý��
��2������c=
�������ҪŨ�����Ũ�ȣ��ٸ�������250mL 0.1mol?L-1��������Һ��Ҫ���Ȼ�������ʵ����������Ҫ�����������250mL��Һ��Ҫ250mL����ƿ��
��3����������ƿ����ȷʹ�÷��������жϣ�
��4�������ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ���ʱӦ��ֱ��ת�Ƶ�ϸ���Լ�ƿ�У�
��5������c=
��������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС��V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ���V������ֵСʱ������ʹ������ҺŨ��ƫ��
��2������c=
| 1000��w |
| M |
��3����������ƿ����ȷʹ�÷��������жϣ�
��4�������ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ���ʱӦ��ֱ��ת�Ƶ�ϸ���Լ�ƿ�У�
��5������c=
| n |
| V |
����⣺��1����Һ©��������ȡ�ͷ�Һ������һ�����ʵ���Ũ����Һ���÷�Һ©������ѡC����ȱ�ٲ���������Ũ����ϡ��ʱ�ò��������裬ת��Һ��ʱ�ò�����������
�ʴ�Ϊ��C�������������裻������
��2����������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�������ʵ���Ũ��Ϊ��c=
=
=12.1��mol/L��������Ũ�������V=
=0.0021L=2.1mL����ѡ��10mL��Ͳ������250mL��Һ��Ӧѡ��250mL������ƿ��
�ʴ�Ϊ��A��C��
��3��A������ƿʹ��ʱ��Ӧ�ȼ���Ƿ�©ˮ��Ȼ��������ˮϴ�Ӹɾ����ɣ���A��ȷ��
B������ƿϴ����������������Һ��ϴ������Ӱ�����Ƶ���Һ��Ũ�ȣ���B����
C������ƿֻ������������Һ������������ƿ���ܽ⣬Ӧ�����ձ����ܽ⣬��C����
D������ƿֻ������������Һ������������ƿ��ϡ�ͣ�Ӧ�����ձ���ϡ�ͣ���D����
E��ҡ��ʱ���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת����E��ȷ��
��ѡAE��
��4������ҡ�Ⱥ�������ƿ�Ŀ̶����Ϸ��в�����Һ��������ҺҺ�����ڿ̶��ߣ�����Ҫר�Ŵ��������Խ����Ƶ���Һֱ��ת�Ƶ�ϸ���Լ�ƿ�У�
��ѡA��
��5��������ƿ������������ˮ��δ���T����Һת�룬��Ӱ��Ũ�ȣ���ѡc��
��ϡ��ŨHClʱ��û����ȴ������ת�Ƶ�����ƿ�У��������Ƶ���Һ���ƫС����ҺŨ��ƫ�ߣ���ѡa��
�����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ�У�������Һ��ϡ�ͣ���Һ��Ũ��ƫ�ͣ���ѡb��
�ܶ���ʱ���������̶ȣ�����Һ��������ڿ̶��ߣ���Һ��������٣�����Ũ��ƫ�ߣ���ѡa��
�ʴ�Ϊ��C�������������裻������
��2����������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�������ʵ���Ũ��Ϊ��c=
| 1000��w |
| M |
| 1000��1.19��37% |
| 36.5 |
| 0.1mol/L��0.25L |
| 12.1mol/L |
�ʴ�Ϊ��A��C��
��3��A������ƿʹ��ʱ��Ӧ�ȼ���Ƿ�©ˮ��Ȼ��������ˮϴ�Ӹɾ����ɣ���A��ȷ��
B������ƿϴ����������������Һ��ϴ������Ӱ�����Ƶ���Һ��Ũ�ȣ���B����
C������ƿֻ������������Һ������������ƿ���ܽ⣬Ӧ�����ձ����ܽ⣬��C����
D������ƿֻ������������Һ������������ƿ��ϡ�ͣ�Ӧ�����ձ���ϡ�ͣ���D����
E��ҡ��ʱ���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת����E��ȷ��
��ѡAE��
��4������ҡ�Ⱥ�������ƿ�Ŀ̶����Ϸ��в�����Һ��������ҺҺ�����ڿ̶��ߣ�����Ҫר�Ŵ��������Խ����Ƶ���Һֱ��ת�Ƶ�ϸ���Լ�ƿ�У�
��ѡA��
��5��������ƿ������������ˮ��δ���T����Һת�룬��Ӱ��Ũ�ȣ���ѡc��
��ϡ��ŨHClʱ��û����ȴ������ת�Ƶ�����ƿ�У��������Ƶ���Һ���ƫС����ҺŨ��ƫ�ߣ���ѡa��
�����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ�У�������Һ��ϡ�ͣ���Һ��Ũ��ƫ�ͣ���ѡb��
�ܶ���ʱ���������̶ȣ�����Һ��������ڿ̶��ߣ���Һ��������٣�����Ũ��ƫ�ߣ���ѡa��
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ŀ�ѶȲ���Ҫ��ѧ��������������һ�����ʵ���Ũ�ȵ���Һ�ķ�������ɷ���������ע��������ɸ���c=
���з����������ʵ����ʵ���n�������ƽ��ƫ�ߣ�����Һ�����V���������Ƶ���ҺŨ��ƫ�ͣ�
| n |
| V |

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ

