��Ŀ����
��24�֣���У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ����(�ܶ�Ϊ1.19 g/cm3)���Ƴ�250mL 0.1mol��L��1��������Һ��
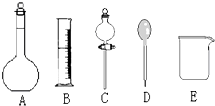
��1������ͼ��ʾ�����У�����������Һ����Ҫ���� ������ͼ��Ӧ��������ţ���
��ͼ�����������⣬����������Һ����Ҫ�IJ��������� �������ð�ʹ�õ��Ⱥ�˳��ֱ���_ ��___ _____��
��2�����ݼ��㣬�������̻����У���ʵ����ͲӦʹ�õ���_______������ƿӦʹ��_______�����ں�������д��Ӧ����ţ������֣���ͬ��
A��10mL B��100mL C��250mL D��500mL
��3��������ƿ��ʹ�÷����У����в�����ȷ���� ��
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ��ˮϴ����������õ�ϡHCl��Һ��ϴ
C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶���
D��������Һʱ����������Һ�壬����Ͳȡ������ֱ�ӵ�������ƿ�У�������ˮ���ӽ��̶���1 ~ 2cm�����ý�ͷ�ιܼ�����ˮ���̶���
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ��
��4�������ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ���ʱӦ��________________
A. ֱ��ת�Ƶ�ϸ���Լ�ƿ�� B. ���ý�ͷ�ιܼ�ˮ�����¶���
C. ������Һ�������������� D. ֱ������������ƿ��
��5����������ʱ������������ȷ��ֻ��������ijһ��������ж������Ƶ���ҺŨ�������Ҫ���ֵ(��0.1 mol/L)��Ρ���a��ƫ�ߣ�b��ƫ�ͣ�c����Ӱ�죬�����к���������Ӧ��ţ�
������ƿ������������ˮ ________
��ϡ��ŨHClʱ��û����ȴ������ת�Ƶ�����ƿ�� ________
�����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ�� ________
��������ʱ���ӣ�������Һ�����ʵ���Ũ�� ________
��1��C ������ ���� ���� ��2��A C
��3��AE ��4��A ��5��c��a��b��a
�������������������Һ��Ҫ��������Ҫ�У���ƽ�����롢Կ�ס��ձ��������塢��Ͳ������ƿ�ͽ�ͷ�ιܣ����Բ���Ҫ���Ƿ�Һ©�����������������ǽ����������
��2��c(HCl)= ������250mL 0.1mol��L��1��������Һ����ҪŨ��������Ϊ
������250mL 0.1mol��L��1��������Һ����ҪŨ��������Ϊ ������ѡ��10mL����Ͳ��250mL����ƿ��
������ѡ��10mL����Ͳ��250mL����ƿ��
��3������ƿ�иǣ�����ʹ��ǰҪ��©������ƿ������ϴ������ƿ��������Һ�������������ܽ�����������Բ���ֱ�ӽ����嵹������ƿ���ܽ⣻������Һ��ҲҪ���ձ���ϡ�ͺ����ת�Ƶ�����ƿ��ҡ��Һ����Ҫ��ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Ρ�������ȷ����AE��
��4��ҡ�Ⱥ���Һ���Ѿ���ã������ٸı����������ֱ�ӽ�Һ�嵹�뵽�Լ�ƿ�С�
��5������c= ��֪������ƿ��������������ˮ�����ʵ�������Һ�������û�иı䣬������Ӱ�졣û����ȴ��ת�Ƶ�����ƿ�У�����Һ��ȴ�����������ֵС������ƫ�ߡ��Լ�ƿ����ˮ����ϡ����õ���Һ������ƫ�͡�����ʱ���ӣ���Һ�����������ֵС������ƫ�ߡ�
��֪������ƿ��������������ˮ�����ʵ�������Һ�������û�иı䣬������Ӱ�졣û����ȴ��ת�Ƶ�����ƿ�У�����Һ��ȴ�����������ֵС������ƫ�ߡ��Լ�ƿ����ˮ����ϡ����õ���Һ������ƫ�͡�����ʱ���ӣ���Һ�����������ֵС������ƫ�ߡ�
���㣺һ�����ʵ���Ũ����Һ������
����������cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�������������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��������ʱ���й������������ݼȿ�����ѡ�������ʽ���п��飬Ҳ��������������ʽ���п��飬�ȿ��Կ����ж����µĽ����Ҳ���Կ����������Ŀ���ԭ��

 ��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�250mL 0.1mol?L-1��������Һ��
��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�250mL 0.1mol?L-1��������Һ��