��Ŀ����
(16��)�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ��ע
��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1 L���ܱ������У�����1mol CO2��3mol H2��һ�������·�����Ӧ��
CO2(g)��3H2(g)  CH3OH(g)��H2O(g)
��H����49.0kJ/mol
CH3OH(g)��H2O(g)
��H����49.0kJ/mol
���CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
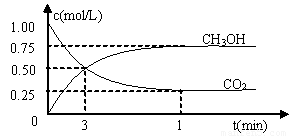
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)��___________mol/(L��min)��������ת����Ϊ ��
�����д�ʩ����ʹn(CH3OH)��n(CO2)�������___________��
A�������¶� B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з��� D���ٳ���1mol CO2��3mol H2
�ƹ�ҵ��Ҳ������CO��H2�ϳɼ״�����Ӧԭ��Ϊ��CO(g)+2H2(g) CH3OH(g) ��H����128.8kJ/mol�����¶Ȳ��������£������������������ͬʱ���ϳ���������ʹH2��Ũ�ȱ��ֲ��䣬��ƽ��
��
CH3OH(g) ��H����128.8kJ/mol�����¶Ȳ��������£������������������ͬʱ���ϳ���������ʹH2��Ũ�ȱ��ֲ��䣬��ƽ��
��
A��������Ӧ�����ƶ� B�����淴Ӧ�����ƶ�
C�����ƶ� D�����ж�
�������жϵ������� ��
��16�֣���1���� 0.225 (3��) 75% (3��) �� C D (4��)
(2) C ��2�֣�
�����ǣ�K��c(CH3OH)/c(CO)c2(H2)��CH3OH��CO�ɱ�����С��c(H2)���䣬��K���� (4��)
��������

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
 CH3OH(g)��H2O(g) ��H����49.0kJ/mol
CH3OH(g)��H2O(g) ��H����49.0kJ/mol
 ���¶� B������He(g)��ʹ��ϵѹǿ����
���¶� B������He(g)��ʹ��ϵѹǿ���� ͬʱ���ϳ���������ʹH2��Ũ�ȱ��ֲ��䣬��ƽ�� ��
ͬʱ���ϳ���������ʹH2��Ũ�ȱ��ֲ��䣬��ƽ�� ��