��Ŀ����
��֪��

�ֽ�A��������ͼ��ʾ�ķ�Ӧ����֪D����Է���������EС��B���ܷ���������Ӧ��F��ʹ��ˮ��ɫ�������к�����
 ��֪
��֪
�Իش��������⣺
��1��A�Ľṹ��ʽ��
��2��д���ٺ͢ڵķ�Ӧ���ͣ���
��3��д�����б仯�Ļ�ѧ����ʽ����F��G��
 ����A��H��
����A��H��
 ��
��
��4��M��A�ķ�������������һ��ͬ���칹�壬��M������
���������Na��Ӧ����������NaHCO3��Ӧ���ܷ���������Ӧ�۲���ʹ��ˮ��ɫ��

�ֽ�A��������ͼ��ʾ�ķ�Ӧ����֪D����Է���������EС��B���ܷ���������Ӧ��F��ʹ��ˮ��ɫ�������к�����
 ��֪
��֪�Իش��������⣺
��1��A�Ľṹ��ʽ��
CH3-CH��OH��-CH2-COOH
CH3-CH��OH��-CH2-COOH
��C��E�Ĺ�ϵ��ͬϵ��
ͬϵ��
����2��д���ٺ͢ڵķ�Ӧ���ͣ���
��ȥ��Ӧ
��ȥ��Ӧ
�����۷�Ӧ
���۷�Ӧ
����3��д�����б仯�Ļ�ѧ����ʽ����F��G��




��4��M��A�ķ�������������һ��ͬ���칹�壬��M������
4
4
�֣����������Na��Ӧ����������NaHCO3��Ӧ���ܷ���������Ӧ�۲���ʹ��ˮ��ɫ��
������F��ʹ��ˮ��ɫ��˵�������к���C=C�������к���������G��֪FΪCH3CH=CHCOOH������A�ܷ�����������ȥ��������Ӧ˵��A��Ӧ����-OH��-COOH����������A���γɰ�Ԫ������AӦΪCH3-CH��OH��-CH2-COOH����������BΪ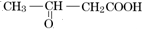 ����CΪHOOC-CH2-COOH��D����Է���������EС����������Ϣ��֪DΪCH3COOH��EΪHOOC-COOH������л���Ľṹ�������Լ������Ϣ�����⣮
����CΪHOOC-CH2-COOH��D����Է���������EС����������Ϣ��֪DΪCH3COOH��EΪHOOC-COOH������л���Ľṹ�������Լ������Ϣ�����⣮
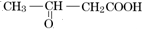 ����CΪHOOC-CH2-COOH��D����Է���������EС����������Ϣ��֪DΪCH3COOH��EΪHOOC-COOH������л���Ľṹ�������Լ������Ϣ�����⣮
����CΪHOOC-CH2-COOH��D����Է���������EС����������Ϣ��֪DΪCH3COOH��EΪHOOC-COOH������л���Ľṹ�������Լ������Ϣ�����⣮����⣺F��ʹ��ˮ��ɫ��˵�������к���C=C�������к���������G��֪FΪCH3CH=CHCOOH������A�ܷ�����������ȥ��������Ӧ˵��A��Ӧ����-OH��-COOH����������A���γɰ�Ԫ������AӦΪCH3-CH��OH��-CH2-COOH����������BΪ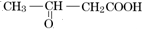 ����CΪHOOC-CH2-COOH��D����Է���������EС����������Ϣ��֪DΪCH3COOH��EΪHOOC-COOH��
����CΪHOOC-CH2-COOH��D����Է���������EС����������Ϣ��֪DΪCH3COOH��EΪHOOC-COOH��
��1�������Ϸ�����֪AΪCH3-CH��OH��-CH2-COOH��CΪHOOC-CH2-COOH��EΪHOOC-COOH����������ͬϵ�
�ʴ�Ϊ��CH3-CH��OH��-CH2-COOH��ͬϵ�
��2����Ӧ��ΪCH3-CH��OH��-CH2-COOH��Ũ���������·�����ȥ��Ӧ����CH3CH=CHCOOH�Ĺ��̣�
��Ӧ��CH3-CH��OH��-CH2-COOH��һ�������·������۷�Ӧ���ɸ߷��ӻ�����Ĺ��̣�
�ʴ�Ϊ����ȥ�����ۣ�
��3��FΪCH3CH=CHCOOH���ɷ����Ӿ۷�Ӧ���ɸ߾����Ӧ�ķ���ʽΪ ��AΪCH3-CH��OH��-CH2-COOH��������A�ɷ���������Ӧ���ɻ�����Ӧ�ķ���ʽΪ
��AΪCH3-CH��OH��-CH2-COOH��������A�ɷ���������Ӧ���ɻ�����Ӧ�ķ���ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��4��AΪCH3-CH��OH��-CH2-COOH����Ӧ��ͬ���칹��M�Т��������Na��Ӧ����������NaHCO3��Ӧ��˵������-OH����������-COOH�����ܷ���������Ӧ��˵������-CHO���۲���ʹ��ˮ��ɫ��˵��������C=C�����Ӧ��ͬ���칹����CH2OHCH2CH2CHO��CH3CHOHCH2CHO��CH3CH2CHOHCHO��CH3COH��CHO��CH34�֣�
�ʴ�Ϊ��4��
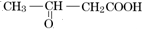 ����CΪHOOC-CH2-COOH��D����Է���������EС����������Ϣ��֪DΪCH3COOH��EΪHOOC-COOH��
����CΪHOOC-CH2-COOH��D����Է���������EС����������Ϣ��֪DΪCH3COOH��EΪHOOC-COOH����1�������Ϸ�����֪AΪCH3-CH��OH��-CH2-COOH��CΪHOOC-CH2-COOH��EΪHOOC-COOH����������ͬϵ�
�ʴ�Ϊ��CH3-CH��OH��-CH2-COOH��ͬϵ�
��2����Ӧ��ΪCH3-CH��OH��-CH2-COOH��Ũ���������·�����ȥ��Ӧ����CH3CH=CHCOOH�Ĺ��̣�
��Ӧ��CH3-CH��OH��-CH2-COOH��һ�������·������۷�Ӧ���ɸ߷��ӻ�����Ĺ��̣�
�ʴ�Ϊ����ȥ�����ۣ�
��3��FΪCH3CH=CHCOOH���ɷ����Ӿ۷�Ӧ���ɸ߾����Ӧ�ķ���ʽΪ
 ��AΪCH3-CH��OH��-CH2-COOH��������A�ɷ���������Ӧ���ɻ�����Ӧ�ķ���ʽΪ
��AΪCH3-CH��OH��-CH2-COOH��������A�ɷ���������Ӧ���ɻ�����Ӧ�ķ���ʽΪ ��
���ʴ�Ϊ��
 ��
�� ��
����4��AΪCH3-CH��OH��-CH2-COOH����Ӧ��ͬ���칹��M�Т��������Na��Ӧ����������NaHCO3��Ӧ��˵������-OH����������-COOH�����ܷ���������Ӧ��˵������-CHO���۲���ʹ��ˮ��ɫ��˵��������C=C�����Ӧ��ͬ���칹����CH2OHCH2CH2CHO��CH3CHOHCH2CHO��CH3CH2CHOHCHO��CH3COH��CHO��CH34�֣�
�ʴ�Ϊ��4��
���������⿼���л�����ƶϣ���Ŀ�ѶȽϴ���ע����FΪͻ�ƿڽ����ƶϣ�ͬʱע�������Ϣ��Ϊ������Ĺؼ����״���Ϊ��4����ע��ͬ���칹����жϣ�

��ϰ��ϵ�д�
�����Ŀ
��֪��
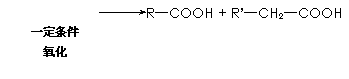 |
![]() R��CH2��C��CH2��R��
R��CH2��C��CH2��R��
|
|
|
|
��֪��A�Ľṹ��ʽΪ��CH3��CH��OH����CH2��COOH ���ֽ�A�������·�Ӧ��B���ܷ���������Ӧ�� D��ʳ����Ҫ�ɷ֣� F�к��м������ҿ���ʹ��ˮ��ɫ��
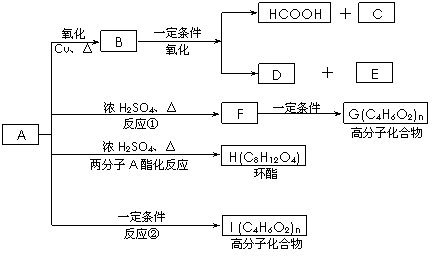 |
��1��д��C��E�Ľṹ��ʽ��C_________ _��E_______ ��
��2����Ӧ�ٺ͢ڵķ�Ӧ���ͣ���____________��Ӧ����____________ ��Ӧ��
��3��д�����л�ѧ����ʽ��
�� F��G��_________________________________________________ ��
�� A��H��_________________________________________________ ��


 +��n-1��H2O
+��n-1��H2O



