��Ŀ����
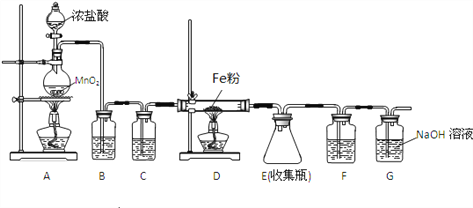
����Ŀ���������ȣ�ClO2)�Ǽ�������ˮ�Ҳ���ˮ������ѧ��Ӧ�Ļ���ɫ���壬�е�Ϊ11�档ijС����ʵ������������ͼ��ʾװ����ȡ���ռ�C1O2���մ��������⣺
(1)C1O2���Ʊ�
��֪:SO2 +2NaClO3 +H2SO4 =2C1O2��+2NaHSO4
��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ_________________________��
�����ռ������C1O2��ѡ����ͼ�е�װ�D��������˳��Ϊa��____________(������������Сд��ĸ��ʾ)��
��װ��D��������_________________��
����û��Eװ�ã�����ɵ����غ����____________________��
(2)ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00mL��ϡ�ͳ�100.00mL��������ȡV1mL�������뵽��ƿ�У�
����2������������pH��2.0������������KI���壬����Ƭ�̣�
����3���������ָʾ������c mol/LNa2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2mL��(��֪2Na2S2O3+I2=Na2S4O6+2NaI)
�ٲ���2�ķ�Ӧ����������ĵ���ʽΪ______��
�ڵζ��յ��ʵ��������_________________��
��ԭC1O2��Һ��Ũ��Ϊ____g/L(�������е���ĸ����ʽ��ʾ)��
�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ⶨ���_______�����ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ��������ⶨ���_____��(�ƫ�ߡ���ƫ�͡����䡱)��
���𰸡� Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O g��h(��h��g)��b��c��e��f��d �������ռ�ClO2 B�е���Һ�ᵹ������A�У������ƿը��
CuSO4+SO2��+2H2O g��h(��h��g)��b��c��e��f��d �������ռ�ClO2 B�е���Һ�ᵹ������A�У������ƿը�� ![]() ��Һǡ������ɫ����ɫ��������30s����
��Һǡ������ɫ����ɫ��������30s���� ![]() ƫ�� ƫ��
ƫ�� ƫ��
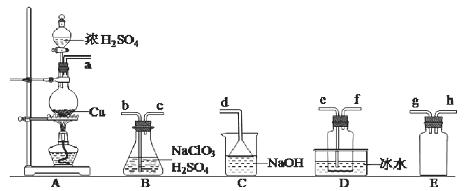
����������1����װ��A��Cu��ŨH2SO4��SO2��װ��A�з�Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O����SO2��a����B��Ϊ��ֹ������Ҫ�а�ȫƿ��a��g��h(��h��g)��Ϊ��ַ�Ӧ��������B�е�b��ClO2�е�ϵͣ�DҪ��ˮԡ���ռ���Ϊ�����ȴ�������ռ�������e�������NaOH����ĩ��Ӧ��SO2������˳��Ϊ g��h(��h��g)��b��c��e��f��d ����ClO2�е�ϵͣ�DҪ��ˮԡ���ռ�����װ��D���������������ռ�ClO2 ����Ϊ��ֹ������Ҫ�а�ȫƿE����û��Eװ�ã�����ɵ����غ����B�е���Һ�ᵹ������A�У������ƿը�� ��(2)����������Ϊ�⣬����ʽΪ
CuSO4+SO2��+2H2O����SO2��a����B��Ϊ��ֹ������Ҫ�а�ȫƿ��a��g��h(��h��g)��Ϊ��ַ�Ӧ��������B�е�b��ClO2�е�ϵͣ�DҪ��ˮԡ���ռ���Ϊ�����ȴ�������ռ�������e�������NaOH����ĩ��Ӧ��SO2������˳��Ϊ g��h(��h��g)��b��c��e��f��d ����ClO2�е�ϵͣ�DҪ��ˮԡ���ռ�����װ��D���������������ռ�ClO2 ����Ϊ��ֹ������Ҫ�а�ȫƿE����û��Eװ�ã�����ɵ����غ����B�е���Һ�ᵹ������A�У������ƿը�� ��(2)����������Ϊ�⣬����ʽΪ![]() �ڵζ��յ��ʵ����������Һǡ������ɫ����ɫ��������30s���䣻
�ڵζ��յ��ʵ����������Һǡ������ɫ����ɫ��������30s���䣻
��3��2ClO2 �� 5I2 �� 10Na2S2O3
2mol 10mol
![]() 1��10-3cV2mol
1��10-3cV2mol
![]()
�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ�����൱�����ĵĵζ�Һ���ƫ�ⶨ���ƫ�����ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ���������൱�����ĵĵζ�Һ���ƫС���ⶨ���ƫС��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�