��Ŀ����
�������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ�����ͼʵ��װ��(����̨���ƾ�����)��֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
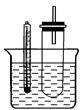
ʵ����������Թ��м���5 mL 1 mol/L NaOH��Һ��5 mL�����飬��
ʵ��������Թ���ͼ�̶���ˮԡ���ȡ�
(1)��ˮԡ���ȶ���ֱ���þƾ��Ƽ��ȵ�ԭ���� ���Թܿڰ�װһ�����ܵ������� ��
(2)�������������Ҵ��Ľṹ�����õIJ����� �� ��
(3)Ϊ֤����������NaOH�Ҵ���Һ�з���������ȥ��Ӧ��������Ƶ�ʵ�鷽���У���Ҫ������� ������ķ����� (��˵�����õ��Լ�����ʵ�������Ԥ�������ʵ������)��

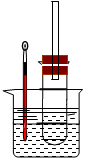
ʵ����������Թ��м���5 mL 1 mol/L NaOH��Һ��5 mL�����飬��
ʵ��������Թ���ͼ�̶���ˮԡ���ȡ�
(1)��ˮԡ���ȶ���ֱ���þƾ��Ƽ��ȵ�ԭ���� ���Թܿڰ�װһ�����ܵ������� ��
(2)�������������Ҵ��Ľṹ�����õIJ����� �� ��
(3)Ϊ֤����������NaOH�Ҵ���Һ�з���������ȥ��Ӧ��������Ƶ�ʵ�鷽���У���Ҫ������� ������ķ����� (��˵�����õ��Լ�����ʵ�������Ԥ�������ʵ������)��
��1��ʹ�Թ����Ⱦ��ȣ��������������ʧ�����������ӷ���������
��2���˴Ź������ס��������
��3�� ���ɵ����壻�����ɵ�������ͨ��ʢ��ˮ���Թܣ���ͨ��ʢ��KMnO4��Һ���Թܣ�KMnO4��Һ��ɫ����ֱ��ͨ��������Ȼ�̼��Һ����ˮ��ɫ����
��2���˴Ź������ס��������
��3�� ���ɵ����壻�����ɵ�������ͨ��ʢ��ˮ���Թܣ���ͨ��ʢ��KMnO4��Һ���Թܣ�KMnO4��Һ��ɫ����ֱ��ͨ��������Ȼ�̼��Һ����ˮ��ɫ����
�����������1��������е�ͣ��ӷ�����ˮԡ�������Ⱦ��ȣ��������������ʧ���������Թܿڰ�װһ���������������ӷ��������飻
��2���Ҵ����ӽṹ����������ԭ�ӣ����ǵı�Ϊ3��2��1�����ú˴Ź������ɼ�⣬Ҳ���ú������⣻
��3�����۷���ȡ����Ӧ������ȥ��Ӧ����Һ�ж������Br-�������ɵ��л��ﲻͬ�������鷢����ȥ��Ӧ������ϩ���壬����Ӧ�������ɵ��л�����ϩ��������ϩ�ɸ�������ʹ����KMnO4��Һ����ˮ��ɫ��ԭ�������У����Բ���ϴ����װ�ã��۲쵽����KMnO4��Һ��ɫ�������ݲ�������ˮ��������Ȼ�̼��Һ��ɫ���ɡ�

��ϰ��ϵ�д�
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ

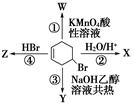
 ��1���� ��CHCl2CHBr2
��1���� ��CHCl2CHBr2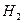 �����ӳɷ�Ӧ���� ( )
�����ӳɷ�Ӧ���� ( )