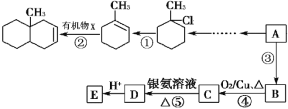
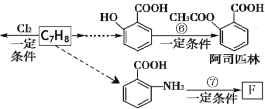
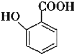
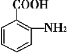
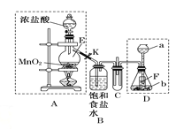
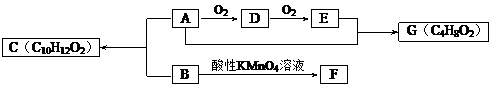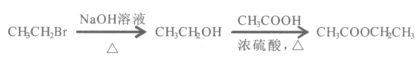��Ŀ����
����Ŀ����һƿ������Һ�����п��ܺ���NH��K����Ba2����Al3����Fe3����I����NO��CO��SO��AlO��ȡ����Һ��������ʵ�飺
����pH��ֽ���飬��Һ��ǿ���ԣ�
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��
����ȡ��Һ��������μ���NaOH��Һ��
a����Һ�����Ա�Ϊ���ԣ�b.��Һ����������c.������ȫ�ܽ⣻d.��������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��ȡ�������õ��ļ�����Һ������Na2CO3��Һ���а�ɫ�������ɡ�
��������ʵ�����ش��������⡣
��1�����������ų�________________________�Ĵ��ڡ�
��2����������֤��________�Ĵ��ڣ�ͬʱ�ų� �Ĵ��ڡ�
��3����������֤��_____________�Ĵ��ڣ�д��c��d���漰�Ļ�ѧ����ʽ�������ӷ�Ӧ�������ӷ���ʽ��ʾ��c________________��d________________��
��4�����������ų�________�Ĵ��ڡ�
���𰸡���1��CO32-��AlO2-����2��I����Fe3����NO3-����3��Al3����NH4+��Al(OH)3��OH��===AlO2-��2H2O��NH3��H2O ![]() NH3����H2O����4��SO42-��
NH3����H2O����4��SO42-��
��������
�������������Һ��ǿ���ԣ�˵���д���H�����ڣ����CO32����AlO2�������ڣ���CCl4����Ϻ�ɫ��˵������I2����Һ����I����û��Fe3�����ڣ���Ϊ���Ƿ���������ԭ��Ӧ��NO3�������������¾���ǿ�����ԣ��ܰ�I��������I2�����Ҳû��NO3��������a���μ�����������Һ�������к�H����b�������������ҳ�����ȫ�ܽ⣬˵��ԭ��Һ�д���Al3��������Al3����3OH��=Al(OH)3����Al(OH)3��OH��=AlO2����2H2O��d�������������������������ʹʪ��ĺ�ɫʯ����ֽ������˵��������ΪNH3��ԭ��Һ�к���NH4����NH4����OH��=NH3��H2O��NH3��H2O![]() NH3����H2O�����õ�������Һ������Na2CO3��Һ��������ɫ�������˳���ֻ����BaCO3��˵��ԭ��Һ����Ba2����һ��������SO42�������ԭ��Һ��һ�����ڵ������ǣ�NH4����Ba2����Al3����I����H����һ�������ڵ�������Fe3����NO3����CO32����SO42����AlO2�������ܴ��ڵ�������K����
NH3����H2O�����õ�������Һ������Na2CO3��Һ��������ɫ�������˳���ֻ����BaCO3��˵��ԭ��Һ����Ba2����һ��������SO42�������ԭ��Һ��һ�����ڵ������ǣ�NH4����Ba2����Al3����I����H����һ�������ڵ�������Fe3����NO3����CO32����SO42����AlO2�������ܴ��ڵ�������K����