��Ŀ����
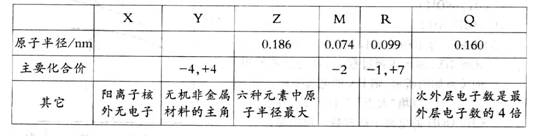
(9��)��x��Y��Z��M��R��Q���ֶ���������Ԫ�أ�������Ϣ���±���ʾ��
��ش��������⣺
(1)R��Ԫ�����ڱ��е�λ����________________________________.
(2)���ݱ��������Ʋ⣬Y��ԭ�Ӱ뾶����С��Χ��_________________________.
(3)Z��M��Q�ļ����ӵ����Ӱ뾶�Ĵ�С˳��Ϊ________________ (��Ԫ�ط��ű�ʾ)��
(4)Y��R��ȣ��ǽ����Խ�ǿ����_________ (��Ԫ�ط��ű�ʾ)��������ʵ��֤����һ��
�۵���__________ (ѡ����ĸ���)��
a��������Y�ĵ��ʳʹ�̬��R�ĵ��ʳ���̬
b���ȶ���XR>YX��
c��Y��R�γɵĻ�������Y������
(5)X��M��Z����Ԫ����ɵĻ������к��еĻ�ѧ��Ϊ________��д��R�ĵ�������
���������ˮ��Һ��Ӧ�����ӷ���ʽ��
__________________________________________________________________________��
��9�֣���1����������VIIA�壨1�֣�
��2������0.099nm С��0.160nm��1�֣�
��3��O2>Na+>Mg2+��1�֣�
��4��C1��1�֣���b.c��ȫ��2�֣��д����÷֣�
��5�����Ӽ������۽���2�֣���Cl2+2OH =Cl-+ClO-+H2O(1��)
=Cl-+ClO-+H2O(1��)
����

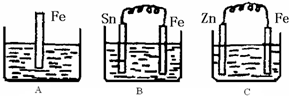
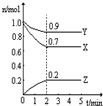
 2Z
2Z ��NaOH��Һ����������ͼ��a��b��c�����ε�ͼ��
��NaOH��Һ����������ͼ��a��b��c�����ε�ͼ��