��Ŀ����
����Ŀ��(1)�й��ĸ����������ڹ������ȵ�λ��
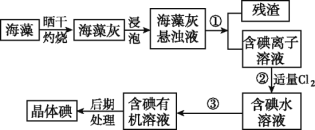
�� ��������������о۰���������ĭ���۰�������_______(����ĸ)��
a���������� b�����ǽ������� c���л��߷��Ӳ���
�� ������Ϣ����ϵͳ��ʹ���˹��ά�����ά����Ҫ�ɷ���_______(����ĸ)��
a��ͭ b��ʯī c����������
�� ��������������ô���ˮ�ࡣ����ˮ�����Ҫԭ��Ϊ�����_________(����ĸ)��
a��ʯ��ʯ b������ c������
(2)Ӫ��ƽ�⡢������ҩ�DZ�֤���彡����������������Ҫ;����
�� ���⡢�ơ���������Ԫ���У��������������������Ԫ�ص���____��(��Ԫ�ط���)
�� ���������У����п���������Ч����____(����ĸ)��
a�������� b����˹ƥ�� c����ù��
�� ��ͼΪijƷ�Ƽ�����ǩ��һ���֣�������ˮ�����ɰ������������_____��������ɫ������_____�����ڷ���������_____��

(3)Ϊ�˼������е�����״������������������ֹȼ���̻�����涨����ȷ�涨����2017��1��1���𣬹�����ȫ�������ݹ�ҵ����������������������������⽭���������������ֹȼ���̻�����
�� ȼú���γ������������������֮һ����ú�м���һ������ʯ��ʯ������____�����������Լ���SO2���ŷš��÷�Ӧ�Ļ�ѧ����ʽΪ_____________________��
�� ����β���к�����Ⱦ������NO��CO�������������ܼ�װ����ת����������ʹCO��NO��Ӧ����������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽΪ____________��
�� ����Ԫ�صķ�ˮ����������ꡣij��ȤС��̽������(Cr2O72��)��ˮ�Ĵ����������ƶ��ķ���������(NH4)2Fe(SO4)2��Cr2O![]() ת��ΪCr3�������ð�ˮ��Cr3��ת������ܵ�Cr(OH)3���ù����е�������Ϊ______���÷���������ķ�ˮ�����д���______(�����ӷ���)���ᵼ��ˮ�帻Ӫ������
ת��ΪCr3�������ð�ˮ��Cr3��ת������ܵ�Cr(OH)3���ù����е�������Ϊ______���÷���������ķ�ˮ�����д���______(�����ӷ���)���ᵼ��ˮ�帻Ӫ������
���𰸡�c c a Fe c ����� ���� �������� ���� 2SO2 + 2CaCO3 + O2 ![]() 2CaSO4 + 2CO2 2CO+2NO
2CaSO4 + 2CO2 2CO+2NO![]() 2CO2 +N2 Cr2O72- NH4+
2CO2 +N2 Cr2O72- NH4+
��������
(1) ���л��߷��ӻ������Ƹ߷��ӻ������߷��ӣ��ֳƸ߾���߷��ӻ�������Է��������ܴ�һ����10000���ϣ�
�ڹ��ά����Ҫ�ɷ��Ƕ������裻
������ˮ�����Ҫԭ���������ʯ��ʯ��
(2) ����Ԫ�ذ��������ܡ�ͭ��п�������̡��⡢�����⡢����
��a.�������ҩ��b.��˹ƥ�־��н�����ʹ���ã�c.��ù�����������ף�
�۸��ݵ�����ˮ�����ɰ�������ʻơ���֬�졢���ܲ��ص��dz��õ���ɫ�������ݱ������ơ�ɽ������dz��õķ�������
(3) ����ú�м���ʯ��ʯ��Ϊ����������Լ���SO2���ŷţ�����CaSO4��
��CO��NO��Ӧ���ɵ����Ͷ�����̼��
�۸÷�����Cr2O72-ת��ΪCr3����CrԪ�ػ��ϼ۽��ͣ���Ӧ�����ɴ���笠����ӡ�
(1) �پ۰������ڸ߷��Ӻϳɲ��ϣ����л��߷��ӻ����
��ˣ�������ȷ���ǣ�c��
�ڹ��ά����Ҫ�ɷ��Ƕ������裬�����ù��ȫ����ԭ����
��ˣ�������ȷ���ǣ�c��
������ˮ�����Ҫԭ���������ʯ��ʯ��
��ˣ�������ȷ���ǣ�a��
(2) �����⡢�ơ���������Ԫ���У��������������������Ԫ�ص�������
��ˣ�������ȷ����:Fe��
�ڰ������ҩ����˹ƥ�־��н�����ʹ���ã���ù��������ҩ��
��ˣ�������ȷ����:c��
�۸��ݼ�����к��е����ʣ�������ˮ�����ɰ����ᣬ��������ɫ��;���������Ƿ���������ˣ�������ȷ���ǣ�����ۣ����ƣ��������ơ�
(3) ����ú�м���ʯ��ʯ��Ϊ����������Լ���SO2���ŷţ�����CaSO4����Ӧ�ķ���ʽΪ��2SO2 + 2CaCO3 + O2 ![]() 2CaSO4 + 2CO2��
2CaSO4 + 2CO2��
��ˣ�������ȷ���ǣ�����2SO2 + 2CaCO3 + O2 ![]() 2CaSO4 + 2CO2��
2CaSO4 + 2CO2��
��CO��NO��Ӧ�Ļ�ѧ����ʽΪ2CO+2NO![]() 2CO2 +N2��
2CO2 +N2��
��ˣ�������ȷ���ǣ�2CO+2NO![]() 2CO2 +N2��
2CO2 +N2��
(3)����(NH4)2Fe(SO4)2��Cr2O72-ת��ΪCr3�������ӷ���ʽΪ��6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O���ù����е�������ΪCr2O72-��������ķ�ˮ�����д���NH4+�ᵼ��ˮ��ĸ�Ӫ������
��ˣ�������ȷ���ǣ�Cr2O72-��NH4+��

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�����Ŀ��X��Y��Z ����ѧ��ѧ�г������������ʣ��±���������֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת����ϵ����
X | Y | Z | ��ͷ���������ֵķ�Ӧ���� | ||
A�� | NO | NO2 | HNO3 | �ٳ��������� |
|
B�� | Cl2 | NaClO | HClO | ��ͨ��CO2 | |
C�� | Na2O2 | NaOH | NaCl | �ۼ���H2O2 | |
D�� | Al2O3 | NaAlO2 | Al(OH)3 | �ܼ�NaOH��Һ |
A. A B. B C. C D. D