��Ŀ����
(08�㽭ʡ������ʮ��У�¿�)���и�����������������ǰ��һ�����ں��ߵ��� ����
�ٴ�ˮ��
��Na+�� F�������Ӱ뾶 �ݺ�1molFeCl3�ı�����Һ��ˮ��ȫ��Ӧת��Ϊ����������������н������ӵ���Ŀ�밢���ӵ�����
A���� �ܡ� ��B��ֻ�Т١� ��C���٢ۢݡ��� D���ڢ�

2(08�㽭ʡ������ѧģ��)ʵ�����и���2SO2��O2![]() 2SO3����H=-393.2 kJ?mol-1�������ͼ��ʾʵ��װ�����Ʊ�SO3���塣��ش��������⡣
2SO3����H=-393.2 kJ?mol-1�������ͼ��ʾʵ��װ�����Ʊ�SO3���塣��ش��������⡣
 |
��1��ʵ��ǰ��������еIJ����ǣ���������ƣ�����д������̣�������������
��2����Aװ���м���Na2SO3�����ͬʱ������Ӽ���ˮ��Ȼ���ٵμ�Ũ���ᡣ�Ӽ���ˮ�������� ��
��3��С�Թ�C��������
��4�����ƿD��ʢ���Լ��� ��װ��D������������ ������ ����
�� ��
��5��ʵ���е�Cr2O3�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȣ��Է��¶ȹ��ߣ���������ԭ���� �� ��
��6��װ��F��U�����ռ��������ʵ���ɫ��״̬��
��7��װ��G��������
��8����Gװ�õ�����β������������
(08����8У����������)1 Lij�����Һ�����ܺ��е��������±���
���ܴ������е������� |
|
���ܴ������е������� |
|
 ��1��������Һ����μ���
��1��������Һ����μ���![]() ��Һ���ʵ�
��Һ���ʵ�
���ȣ�������������������ʵ�����![]() ��
��
�����![]() ��Һ�������V���Ĺ�ϵ
��Һ�������V���Ĺ�ϵ
����ͼ��ʾ�������Һ��ȷ�����е�����
��_______________������ȷ���Ƿ���
����������______________��Ҫȷ�����
�ڿɲ�������ʵ����___________���϶������ڵ���������_________________��
��2������⣬����Һ�к��д�����![]() ������1 L�û����Һ��ͨ��D���ε�
������1 L�û����Һ��ͨ��D���ε�![]() ����Һ��
����Һ��![]() �����ʵ�����ͨ��
�����ʵ�����ͨ��![]() ���������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
���������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺

 (08������Ϣ��)2008�걱�����˻����֡����İ��ˡ���һ����Ҫ�ٴ��Ǽ�������˶�Ա�����˷ܼ�����������һ�ֱ���ֹʹ�õ�ҩ���ṹ��ʽ��ͼ��ʾ�������й�������˵����ȷ���ǣ���
(08������Ϣ��)2008�걱�����˻����֡����İ��ˡ���һ����Ҫ�ٴ��Ǽ�������˶�Ա�����˷ܼ�����������һ�ֱ���ֹʹ�õ�ҩ���ṹ��ʽ��ͼ��ʾ�������й�������˵����ȷ���ǣ���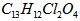
 ��Һ������ɫ��Ӧ
��Һ������ɫ��Ӧ ������˵����ȷ���ǣ���
������˵����ȷ���ǣ��� A��a��Ϊ��ص�����
A��a��Ϊ��ص�����
 ���صĵ���
���صĵ���  ��CH3OH��g��+H2O��g��=CO2��g��+3H2��g����H1=+49.0KJ/mol
��CH3OH��g��+H2O��g��=CO2��g��+3H2��g����H1=+49.0KJ/mol