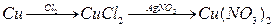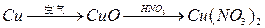��Ŀ����
��12�֣�ijͬѧ����ʵ���������Fe��ˮ������Ӧ��ʵ�飬װ����ͼ�ס������֡�
��֪B�з����������ʯ���Ļ���C�зŵ��Ǹ������EΪ�ƾ���ƣ�GΪ������˿���ֵľƾ��ơ�
��.�Ա���װ�ã��ش��������⣺
��1����μ����װ�õ������ԣ� ��
��2����װ����ʪɳ�ӵ������� ��
��3��B��������Ӧ�Ļ�ѧ����ʽ�� ��
��4����ͬѧ��Ϊ������ װ�õļ��쵼�ܴ���ȼ��Ӧ���������壬װ��H�ز����٣�H�������� ��
װ�õļ��쵼�ܴ���ȼ��Ӧ���������壬װ��H�ز����٣�H�������� ��
��5���Աȼס�����װ�õ�B��K��B���ŵ��� ��
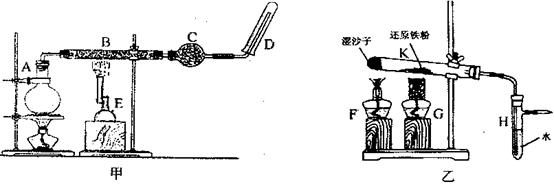
��.ijͬѧΪ���о�һ��ʱ�������۵�ת���ʣ�����ͼ�еļ�װ�����������ʵ�飺ȷ����һ�����������۽��з�Ӧ���ռ���������Ӧ�����ɵ�����������������۵�ת���ʡ�
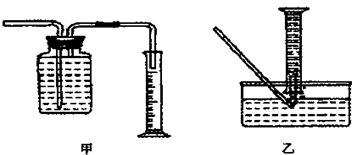
������ˮ���ռ����������������ѡ��ͼ�е� װ�á�
�ڳ�ȡmg����������ʯ����ϣ�Ȼ�������������ų������ռ��������������Ϊ��״��ΪVL�����۵�ת����Ϊ���г�����ʽ���ɣ� ��
��Ϊ��֤����Ӧ��Ĺ��������к���+3�۵�Fe����ͬѧȡ��������������Թ��У��������������ᣬ�ܽ����ˣ���������Һ�еμ�KSCN��Һ������۲쵽��Һ����ɫû�仯������˼������ͬѧ��Ϊ��������˵����Ӧ��Ĺ��������в�����+3��Fe�����������ǣ� ��

��֪B�з����������ʯ���Ļ���C�зŵ��Ǹ������EΪ�ƾ���ƣ�GΪ������˿���ֵľƾ��ơ�
��.�Ա���װ�ã��ش��������⣺
��1����μ����װ�õ������ԣ� ��
��2����װ����ʪɳ�ӵ������� ��
��3��B��������Ӧ�Ļ�ѧ����ʽ�� ��
��4����ͬѧ��Ϊ������
 װ�õļ��쵼�ܴ���ȼ��Ӧ���������壬װ��H�ز����٣�H�������� ��
װ�õļ��쵼�ܴ���ȼ��Ӧ���������壬װ��H�ز����٣�H�������� ����5���Աȼס�����װ�õ�B��K��B���ŵ��� ��
��.ijͬѧΪ���о�һ��ʱ�������۵�ת���ʣ�����ͼ�еļ�װ�����������ʵ�飺ȷ����һ�����������۽��з�Ӧ���ռ���������Ӧ�����ɵ�����������������۵�ת���ʡ�
������ˮ���ռ����������������ѡ��ͼ�е� װ�á�

�ڳ�ȡmg����������ʯ����ϣ�Ȼ�������������ų������ռ��������������Ϊ��״��ΪVL�����۵�ת����Ϊ���г�����ʽ���ɣ� ��
��Ϊ��֤����Ӧ��Ĺ��������к���+3�۵�Fe����ͬѧȡ��������������Թ��У��������������ᣬ�ܽ����ˣ���������Һ�еμ�KSCN��Һ������۲쵽��Һ����ɫû�仯������˼������ͬѧ��Ϊ��������˵����Ӧ��Ĺ��������в�����+3��Fe�����������ǣ� ��
��12�֣�
��.��1����H�м���ˮû�����ܿڣ����Ӻ�װ�ã���K����H�е��ܿڳ���
���ݣ�ֹͣ���Ⱥ��г���ˮ����֤�����������ã�����������Ҳ���֣���2�֣�
��2���ṩˮ������1�֣���3��3Fe+4H2O==Fe2O3+4H2��2�֣���4������ˮ������1�֣�
��5��ʹ��ʯ���ޣ�����ˮ������Fe�۽Ӵ�������ӿ췴Ӧ���ʣ�1�֣�
��.���ң�1�֣�
��
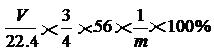 ��2�֣���ע�����������ȷ�Ĵ���ʽ��
��2�֣���ע�����������ȷ�Ĵ���ʽ�� �ɣ���
�ɣ���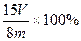 ��
��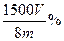 ��
��
�����ۿ�����ʣ�࣬ʣ�����ۻ���Fe3+��Ӧ����Fe3+��Һ����ȫ��ԭΪFe2+����2�֣�
��.��1����H�м���ˮû�����ܿڣ����Ӻ�װ�ã���K����H�е��ܿڳ���
���ݣ�ֹͣ���Ⱥ��г���ˮ����֤�����������ã�����������Ҳ���֣���2�֣�
��2���ṩˮ������1�֣���3��3Fe+4H2O==Fe2O3+4H2��2�֣���4������ˮ������1�֣�
��5��ʹ��ʯ���ޣ�����ˮ������Fe�۽Ӵ�������ӿ췴Ӧ���ʣ�1�֣�
��.���ң�1�֣�
��

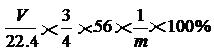 ��2�֣���ע�����������ȷ�Ĵ���ʽ��
��2�֣���ע�����������ȷ�Ĵ���ʽ�� �ɣ���
�ɣ���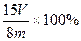 ��
��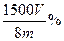 ��
�������ۿ�����ʣ�࣬ʣ�����ۻ���Fe3+��Ӧ����Fe3+��Һ����ȫ��ԭΪFe2+����2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
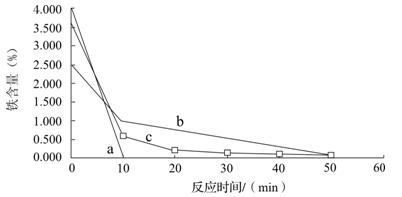
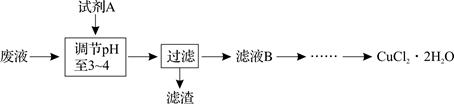
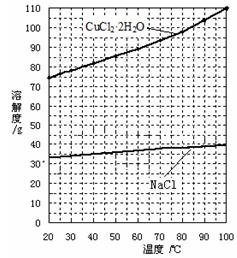
 ����ͭ
����ͭ ����ͭ
����ͭ