��Ŀ����
����������ֳ�Ī���Σ�dz��ɫ���壬�ڿ����б�һ����������ȶ�������ˮ���������Ҵ�����ѧʽΪ[��NH4��2SO4?FeSO4?6H2O]����Է�������Ϊ392���dz��õķ����Լ�����ʵ���ң���FeSO4�ͣ�NH4��2SO4������Һ��һ��������ϣ�����Ũ������ȴ�ᾧ�������õ���������茶��壮ij�о���ѧϰС�����Ʊ��õ�Ī���Σ��������о����ǵõ��IJ�Ʒ�����������ɲ�����Ŀ��
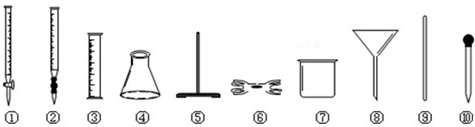
��1��Ԥ��������ʢ��Ī������Һ�Ĵ��Թ��еμ�ŨNaOH��Һ����������
��2��Ϊ��ȷ����Ʒ�������������о�С�龭�������Ϻ�����KMnO4���ữ����Һ�ζ�Ī������Һ�е�Fe2+�����ӷ���ʽΪ��5Fe2++MnO4-+8H+=5Fe2++Mn2++4H2O���ζ�ʱ����ѡ�õ�������
��3��ȡĪ���β�Ʒ23.520g�����250mL��Һ��ȡ��25.00mL��0.0500mol/LKMnO4��Һ�ζ�������KMnO4��Һ19.50mL�����Ʒ��Ī���ε���������
��1��Ԥ��������ʢ��Ī������Һ�Ĵ��Թ��еμ�ŨNaOH��Һ����������
���ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ��ͬʱ�ų������̼�������ʹ��ɫʯ����ֽ����������
���ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ��ͬʱ�ų������̼�������ʹ��ɫʯ����ֽ����������
����2��Ϊ��ȷ����Ʒ�������������о�С�龭�������Ϻ�����KMnO4���ữ����Һ�ζ�Ī������Һ�е�Fe2+�����ӷ���ʽΪ��5Fe2++MnO4-+8H+=5Fe2++Mn2++4H2O���ζ�ʱ����ѡ�õ�������
�٢ܢݢ�
�٢ܢݢ�
������ͼ����������ѡ���ţ�ͬ�������������ޣ����ζ�ʱ�Ƿ���Ҫ�Ӽ�ָʾ��������Ҫ
����Ҫ
������Ҫ����ָ����ʲôָʾ����������Ҫ����˵�����ɣ�Fe2+��ȫ��Ӧ�������ĸ�����ؽ�ʹ��Һ������ɫ����ָʾ�յ�
Fe2+��ȫ��Ӧ�������ĸ�����ؽ�ʹ��Һ������ɫ����ָʾ�յ�
��
��3��ȡĪ���β�Ʒ23.520g�����250mL��Һ��ȡ��25.00mL��0.0500mol/LKMnO4��Һ�ζ�������KMnO4��Һ19.50mL�����Ʒ��Ī���ε���������
81.3%
81.3%
����������1������Ī���λ�ѧʽ[��NH4��2SO4?FeSO4?6H2O]���ܹ�����ˮ�����ʽ��з�����
��2��ע���ǵζ�ʱѡ�õ�����������������Һ��Ҫ����������Ӧ��ѡ����ʽ�ζ��ܡ���ƿ������̨�����У�Fe2+��ȫ��Ӧ�������ĸ�����ؽ�ʹ��Һ������ɫ����ָʾ�յ㣻
��3���������ĵĸ�����ص����ʵ�����������������ӵ����ʵ������ټ����250mL��Һ�к��е�Ī���ε��������������Ī���ε�����������
��2��ע���ǵζ�ʱѡ�õ�����������������Һ��Ҫ����������Ӧ��ѡ����ʽ�ζ��ܡ���ƿ������̨�����У�Fe2+��ȫ��Ӧ�������ĸ�����ؽ�ʹ��Һ������ɫ����ָʾ�յ㣻
��3���������ĵĸ�����ص����ʵ�����������������ӵ����ʵ������ټ����250mL��Һ�к��е�Ī���ε��������������Ī���ε�����������
����⣺��1��Ī���λ�ѧʽ�к����������Ӻ�����ӣ��ܹ����������Ʒ�Ӧ���������������Ͱ�����������ʢ��Ī������Һ�Ĵ��Թ��еμ�ŨNaOH��Һ������Ϊ�����ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ��ͬʱ�ų������̼�������ʹ��ɫʯ����ֽ���������壬
�ʴ�Ϊ�����ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ��ͬʱ�ų������̼�������ʹ��ɫʯ����ֽ���������壻
��2�����������Һ����ǿ�����ԣ�Ӧ��ʹ�â���ʽ�ζ��ܣ�ʢװ����Һ��Ҫʹ�â���ƿ���ζ�����Ҫ�̶����õ�������̨�������У�����Fe2+��ȫ��Ӧʱ�������ĸ�����ؽ�ʹ��Һ������ɫ����ָʾ�յ㣬����Ҫ����ָʾ����
�ʴ�Ϊ���٢ܢݢޣ�����Ҫ�� Fe2+��ȫ��Ӧ�������ĸ�����ؽ�ʹ��Һ������ɫ����ָʾ�յ㣻
��3�����ĵĸ�����ص����ʵ���Ϊ��0.0500mol/L��0.0195L=9.75��10-4mol��
250mL��Һ����Ҫ���ĵĸ�����ص����ʵ���Ϊ��9.75��10-4mol��
=9.75��10-3mol��
���ݷ�Ӧ5Fe2++MnO4-+8H+=5Fe2++Mn2++4H2O��
�������ӵ����ʵ���Ϊ��n��Fe2+��=5n��MnO4-��=5��9.75��10-3mol=4.875��10-2mol��
Ī���β�Ʒ23.520g�к��е�Ī��������Ϊ��392g/mol��4.875��10-2mol=19.11g��
��Ʒ��Ī���ε���������Ϊ��
��100%��81.3%��
�ʴ�Ϊ��81.3%��
�ʴ�Ϊ�����ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ��ͬʱ�ų������̼�������ʹ��ɫʯ����ֽ���������壻
��2�����������Һ����ǿ�����ԣ�Ӧ��ʹ�â���ʽ�ζ��ܣ�ʢװ����Һ��Ҫʹ�â���ƿ���ζ�����Ҫ�̶����õ�������̨�������У�����Fe2+��ȫ��Ӧʱ�������ĸ�����ؽ�ʹ��Һ������ɫ����ָʾ�յ㣬����Ҫ����ָʾ����
�ʴ�Ϊ���٢ܢݢޣ�����Ҫ�� Fe2+��ȫ��Ӧ�������ĸ�����ؽ�ʹ��Һ������ɫ����ָʾ�յ㣻
��3�����ĵĸ�����ص����ʵ���Ϊ��0.0500mol/L��0.0195L=9.75��10-4mol��
250mL��Һ����Ҫ���ĵĸ�����ص����ʵ���Ϊ��9.75��10-4mol��
| 250 |
| 25 |
���ݷ�Ӧ5Fe2++MnO4-+8H+=5Fe2++Mn2++4H2O��
�������ӵ����ʵ���Ϊ��n��Fe2+��=5n��MnO4-��=5��9.75��10-3mol=4.875��10-2mol��
Ī���β�Ʒ23.520g�к��е�Ī��������Ϊ��392g/mol��4.875��10-2mol=19.11g��
��Ʒ��Ī���ε���������Ϊ��
| 19.11g |
| 23.520g |
�ʴ�Ϊ��81.3%��
���������⿼����Ī���εĺ����ⶨ��ע��������Ϣ�Ĵ�����������ѧ֪ʶ��ɼ��ɣ������Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��ʵ�黯ѧ��
��ʵ�黯ѧ��