��Ŀ����
����Ŀ��L��M��R��T��W��ԭ��������������Ķ���������Ԫ�أ�M��T�����ڱ��е����λ�����±���L��Rԭ�ӵ�������������ͬ��R�ĵ�����һ�ֳ����İ뵼����ϡ�
M | |
T |
��ش��������⣺
(1)T���ӵĽṹʾ��ͼΪ__________��Ԫ��W�����ڱ���λ�ڵ�______�壬M���ʷ��ӵĵ���ʽΪ__________��
(2)R����ɲ�����Ԫ��֮һ�������ô�ĥ�ڲ��������Լ�ƿʢ������������Һ��ԭ����(�û�ѧ����ʽ��ʾ)__________��
(3)�����й���ӦԪ�طǽ�����ǿ���Ƚϵ�˵������ȷ����(�����)__________��
a��M����̬�⻯���R����̬�⻯���ȶ�����ǽ�����Mǿ��R
b�������£�L�ĵ����ܴ�R��������������û���R����ǽ�����Lǿ��R
c��W�ĺ˵������T�࣬ԭ�Ӱ뾶��TС���õ�������ǿ����ǽ�����Wǿ��T
(4)��ҵ�Ͽ��ö������衢�����ͽ�̿�ڸ����������Ʊ�RW4���÷�Ӧ�г�RW4�����һ�ֲ�������ڹ�ҵ�������Ʊ�RW4�Ļ�ѧ����ʽΪ______________________________��
���𰸡� 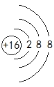 ��A
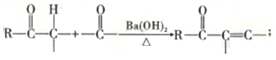
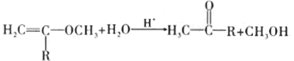
��A ![]() SiO2+2NaOH==Na2SiO3+H2O ac 2Cl2+SiO2+2C
SiO2+2NaOH==Na2SiO3+H2O ac 2Cl2+SiO2+2C![]() SiCl4+2CO
SiCl4+2CO
�����������������R�ĵ�����һ�ֳ����İ뵼����ϣ�R��SiԪ�أ�L��Rԭ�ӵ�������������ͬ��L��CԪ����L��M��R��T��W��ԭ��������������������M��T�����ڱ��е����λ�ã�M�Ǣ�A��Ԫ�ء�T�Ǣ�A��Ԫ�ء�W�Ǣ�A��Ԫ�أ�����M��T��W�ֱ���N��S��ClԪ����
�������������Ϸ�����(1)S2-�Ľṹʾ��ͼΪ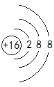 ����Ԫ�������ڱ���λ�ڵ�3���ڣ��ڢ�A����N2�����д��ڵ�������������ʽΪ
����Ԫ�������ڱ���λ�ڵ�3���ڣ��ڢ�A����N2�����д��ڵ�������������ʽΪ![]() ��
��
(2)�����������������Ʒ�Ӧ���ɹ����ƺ�ˮ�����Բ����ô�ĥ�ڲ��������Լ�ƿʢ������������Һ����ѧ����ʽΪSiO2+2NaOH==Na2SiO3+H2O��
(3)�����й���ӦԪ�طǽ�����ǿ���Ƚϵ�˵������ȷ����(�����)__________��
NH3��SiH4�ȶ�����ǽ�����Nǿ��Si����a��ǿ��
�����µ���̼�ܴӶ����������û�����������̼�Ļ�ԭ�ԣ������Ƴ��ǽ�����Cǿ��Si����b������
�õ�������Խǿ���ǽ�����Խǿ����c��ȷ��
(4)��ҵ�Ͽ��ö������衢�����ͽ�̿�ڸ�������������SiCl4��CO���Ʊ�SiCl4�ķ�Ӧ����ʽΪ2Cl2+SiO2+2C![]() SiCl4+2CO��
SiCl4+2CO��

����Ŀ���Ȼ�ѧ����ƽ��ȶ��ǻ�ѧ�о��Ķ���
(1)��֪����2O2(g)+N2(g)==N2O4(l) ��H1��
��N2(g)+2H2(g)==N2H4(g) ��H2��
��O2(g)+2H2(g)==2H2O(g) ��H3��
��2N2H4(g) +N2O4(1)==3N2(g)+4H2O(g) ��H4��
����������ЧӦ֮��Ĺ�ϵʽΪ��H4 =_______(�ú���H1����H2����H3�Ĵ���ʽ��ʾ����
(2)����ͬ����H2O(g)��CO(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У����з�Ӧ��H2O(g)+CO(g) ![]() H2(g)+CO2(g)���õ����±��������ݣ�
H2(g)+CO2(g)���õ����±��������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ�� ����ʱ��/min | ||
H2O | CO | H2 | CO | |||
1 | 800 | 2 | 4 | 4/3 | 8/3 | 6 |
2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
3 | 900 | a | b | c | d | t |
�ٸ÷�Ӧ���淴ӦΪ______(����ȡ����ȡ�����Ӧ��ʵ��2��ƽ�ⳣ��K=________��
����ʵ��3�ﵽƽ��ʱ��ʵ��2�ﵽƽ��״̬ʱ�����ʵ���������ֱ���ȣ���t<3����a��bӦ����Ĺ�ϵ��_______(�ú�a��b�Ĵ���ʽ��ʾ����
���������¶Ⱥ��ݻ����䣬��ʵ��1������4molH2O(g)��ʹ��Ӧ�ﵽ�µ�ƽ�⡣����˵������ȷ����______������ţ���
A.�¡���ƽ��ʱ�����������ѹǿ֮����5 : 3
B.��ƽ��ʱH2O��ת��������
C.��ƽ��ʱCO��Ũ����0.8 mol��L-l
D.�¡���ƽ��ʱ�����������ܶ�֮��Ϊ5 : 3
(3)�����£���0.1 mol��L-l��KOH��Һ�ζ�10.00Ml0.10 mol��L-l H2C2O4 (��Ԫ���ᣩ��Һ�����õζ�������ͼ(�����Һ������ɿ��ɻ��ǰ��Һ�����֮��)����ش��������⣺
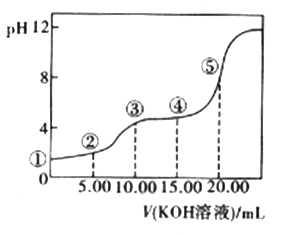
a.�����ʾ��Һ�еĵ���غ�ʽΪ________________________��
b.�����ʾ��Һ���������ӵ�Ũ���ɴ�С��˳��Ϊ___________________��
c.�ɢ���ʾ��Һ��c(K+)+c(H2C2O4)+c(HC2O4-)+c(C2O42-)=________mol/L��