��Ŀ����
ˮ�ϼ�ʽ̼��þ[4MgC03��Mg(OH)2��4H2O�����ֳ�����̼��þ����������þ��(��������Ϊ̼��þ90% ��̼���10%��̼������ȡ��

��1���������б������ݣ�ѡ��������Ӧ������¶�_____��������__________��

��2��̼����Ӧ������Mg(HCO3)2������Mg(HCO3)2�Ļ�ѧ����ʽΪ_______��
��3������ͼ��______��_____����Ϊ̼����Ӧ�ṩ������̼Դ��
��4���й����������±ˮ̼������ȡ����̼��þ��
��±ˮ�к���Fe2+��Mn2+������Ũ��С��1��10-5ʱ��������Ϊ��ȫ��ȥ������ʱ��������ҺpHΪ9.5ʱ����ʱMn2+С��_____mol/L���������������
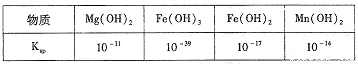
�������Fe2+ת��ΪFe3+���ӻ����Ƕ�ѡ������ʵ�������Ϊ______��
A. Ca(C10)2 B. Cl2 C. H202 D. HNO3
�����з����У��Ϻõ�Ϊ_______��������___________��

����﮵��������������ﮣ�LiMn2O4)����ȡ���㷺ʹ�õ�LiCoO2����ҵ����ij���̿�(��Ҫ�ɷ�ΪMnO2��ͬʱ������������������ȵ������Ϊԭ���Ʊ�����﮵��������£�
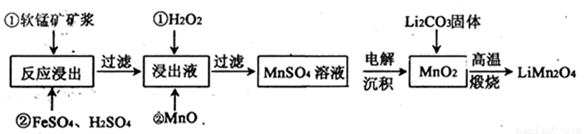
�й����ʵ��ܶȻ�����
���� | Fe(OH)2 | Fe(OH)3 | Al(OH)3 | Mn(OH)2 |
Ksp | 8.0��10-16 | 4.0��l0-38 | 4.5��10-33 | 1.9��l0-13 |
(1)��֪��﮵�طŵ������ĵ缫��ӦΪ��LiMn2O4+e-+Li+= Li2Mn2O4����﮵����������������У���Ԫ�صĻ��ϼ�Ϊ________��
(2)�����У�FeSO4��������_______��MnO��������_________������Һ�е�pHΪ6ʱ����Һ�����������ӵ�Ũ��Ϊ___________��
(3)������ͼװ�õ�⣬���ӽ���Ĥ�����طָ�Ϊ�����Һ������ң����ҵ���Һ�ֱ�Ϊ��������Һ���Ƶõ���������Һ�����������е���ҺΪ________����������MnO2�����ڵ缫�ϣ��õ缫��ӦʽΪ_________��
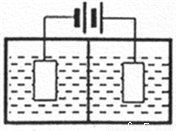
(4)��������װ���е���������Һ��Ϊ���̿�Ŀ������������������������ᣬ��һ����������̿�Ľ�����Ӧ�������MnO2��Ӧ�����ʱ��Fe3+�ȷŵ�����Fe2+��������Fe2+������е� MnO2��Ӧ���ܶ���ʼ��ֱ�����е�MnO2��ȫ��������Fe2+�����MnO2��Ӧ�����ӷ���ʽΪ__________��
(5)д������������������﮵Ļ�ѧ����ʽ___________��
���г�������ȷ�����������ϵ����
ѡ�� | ����I | ������ |
A | HClO����ǿ������ | HClO��ɱ���������� |
B | ���������� | �����������ױ���ʴ |
C | ��ˮ��Ӧʱ���Ƹ���ˮ���� | ����ˮ��Ӧ�Ƿ��ȷ�Ӧ |
D | Ũ�������ǿ������ | Ũ�����ܺ�Ũ��ˮ��Ӧ�������� |
A. A B. B C. C D. D
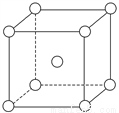
«���е��춬����(�ṹ��ͼ)����Ԫ�����������̵ȣ�������������������Ĺ�Ч��
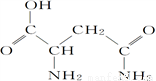
(1)�춬��������Ԫ���У�________(��Ԫ�����ƣ���ͬ)Ԫ�ػ�̬ԭ�Ӻ���δ�ɶԵ�������࣬��һ������������________��
(2)�춬������̼ԭ�ӵ��ӻ��������Ϊ________�������ЦҼ��ͦм���Ŀ֮��Ϊ________��
(3)O��S��SeΪͬ����Ԫ�أ�H2O��H2S��H2Se�IJ����Աȼ�����
��ѧʽ | ����/nm | ���� |
H2O | 0.99 | 104.5�� |
H2S | 1.34 | 92.3�� |
H2Se | 1.47 | 91.0�� |
H2S�ļ��Ǵ���H2Se��ԭ�����Ϊ________________________________________��
H2O��H2S��H2Se�е��ɸߵ��͵�˳��Ϊ________________��������ǿ������˳��Ϊ________________��
(4)д�����Ļ�̬ԭ�ӵ����Ų�ʽ��________________________________________��
(5)��Ϊ�����������壬�����ṹ��ͼ����þ����к���______����ԭ�ӡ��������ܶ�Ϊ��g��cm��3�����ԭ������ΪM��NA��ʾ�����ӵ�������ֵ�����ԭ�ӵİ뾶Ϊ______cm��



 MnO(s)+CO2(g) ��H1 K1
MnO(s)+CO2(g) ��H1 K1