��Ŀ����
����Ŀ����������2017����ѧ�ڵ�����������̼�������仯������ͬѧ�Ǿ����ܽӴ�������Ҫ���ʣ��ǿ�ѧ�о�����Ҫ����
��1��ʵ������ȡ��Ȳ�Ļ�ѧ����ʽΪ___________________________��
��2��H2NCOONH4�ǹ�ҵ�ϳ����ص��м����÷�Ӧ�������仯��ͼA��ʾ����CO2�Ͱ����ϳ����ص��Ȼ�ѧ����ʽΪ___________________________��
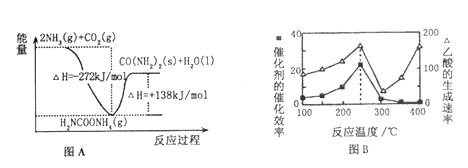
��3����������CO2��CH4����������ЧӦ��Ϊ��ѧ�о������ȵ㡣һ���Զ������ѱ��渲��Cu2A12O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᣨ��H<0)���ڲ�ͬ�¶��´����Ĵ�Ч����������������ʷֱ�����ͼB��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ����________________��250����400��ʱ������������ʼ�����ȣ�ʵ��������Ӧѡ����¶�Ϊ_________����
��4��T��ʱ���������ʵ�����NO��CO�������Ϊ2L�� �ܱ������з�����Ӧ2NO+2CO![]() 2CO2+N2�������¶Ⱥ�������䣬��Ӧ������NO�����ʵ�����ʱ��ı仯��ͼC��ʾ��
2CO2+N2�������¶Ⱥ�������䣬��Ӧ������NO�����ʵ�����ʱ��ı仯��ͼC��ʾ��
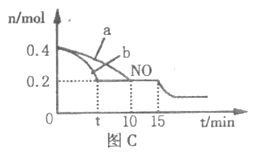
��ƽ��ʱ�������¶Ȳ��䣬���������г���CO��N2��0.8mol��ƽ�⽫______(����������ҡ��������ƶ���
��ͼ��a��b�ֱ��ʾ��һ���¶��£�ʹ����ͬ��������ͬ������Ĵ���ʱ���ﵽƽ�������n(NO)�ı仯���ߣ����б�ʾ����������ϴ��������______(�a����b��)��
��15minʱ,���ı���練Ӧ����������n(NO)������ͼ��ʾ�ı仯����ı������������_____________(�δ�һ������)��
��5����������Һ�к��д����İ������ʣ���NH3��ʾ�����Ȼ�����õ��ԭ������Һ�еİ���������ȫ������ȥ���ù��̷�Ϊ��������һ�����������������ڶ���������������������������ΪN2��
���ڶ�����Ӧ�Ļ�ѧ����ʽΪ____________________��
������������Һ�а������ʵ���������Ϊ0��034% ���������õ�ⷨ����It����ˮ����·��ת�Ƶĵ�����Ϊ__________��
���𰸡�CaC2 + 2H2O��Ca(OH)2 + C2H2�� 2NH3(g) + CO2(g)��CO(NH2)2(s)+ H2O��1����H=-134 kJ/mol 250���������Ĵ�Ч����ã�֮������Ĵ�Ч�ʼ��罵�� 250�� ���� b ����CO�����ʵ���Ũ�ȡ�����ѹǿ������������Ũ�� 3Cl2+2NH3==N2+6HCl 3.612��1025 (��60NA)
����������1��ʵ�����õ�ʯ��ˮ��Ӧ��ȡ��Ȳ����ѧ����ʽΪ ��1����CaC2 + 2H2O = Ca(OH)2 + C2H2����
��2����Ӧ�������ߣ������������ͣ���ͼ��֪�ų�������Ϊ��272kJ/mol -138kJ/mol =134kJ/mol ����CO2�Ͱ����ϳ����ص��Ȼ�ѧ����ʽΪ2NH3(g) + CO2(g)==CO(NH2)2(s)+ H2O��1����H=-134 kJ/mol ��3��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ���ǣ�250���������Ĵ�Ч����ã�֮������Ĵ�Ч�ʼ��罵�ͣ�250����400��ʱ������������ʼ�����ȣ�ʵ��������Ӧѡ����¶�Ϊ250����250��ʱ����������ߡ���4����ʼNOΪ0.4molƽ��ʱΪ0.2mol

ƽ��ʱŨ��Ϊ0.1mol/L��0.1mol/L��0.1mol/L��0.05mol/L
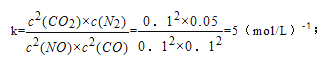
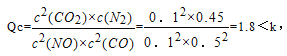 �ʷ�Ӧ���ҽ��С�
�ʷ�Ӧ���ҽ��С�
�������������Ӧ���ʿ죬��ƽ��ʱ��̣���ͼ��֪��b���ߴ��������µķ�Ӧ���ʿ죬b�Ĵ��������������ͼ���֪��NO��Ũ�ȼ�С��ƽ�������ƶ������Ըı�����Ϊ����CO�����ʵ���Ũ�ȡ�����ѹǿ������������Ũ�� ��
��5���ڶ���������������������������ΪN2������ʽΪ��3Cl2+2NH3==N2+6HCl��n(NH3)=106g��0��034%/17g��mol-1==20mol,N��-3�۱��0�ۣ�ת�Ƶ�����3.612��1025 (��60NA)��

����Ŀ��������ʡ2017�꿼ǰ������������������β�����ŷŵ�NOx��CO��Ⱦ������������β��ϵͳ��װ�ô�ת����������Ч����NOx��CO���ŷš�
��֪����2CO(g)��O2(g) ![]() 2CO2(g) ��H��566.0 kJ��mol1
2CO2(g) ��H��566.0 kJ��mol1
��N2(g)��O2(g) ![]() 2NO(g) ��H��+180.5 k J��mol1
2NO(g) ��H��+180.5 k J��mol1
��2NO(g)��O2(g) ![]() 2NO2(g) ��H��116.5 k J��mol1
2NO2(g) ��H��116.5 k J��mol1
�ش��������⣺
��1��CO��ȼ����Ϊ_________����1 mol N2(g)��1 mol O2(g) �����л�ѧ������ʱ�ֱ���Ҫ����946 kJ��498 kJ����������1 mol NO(g) �����л�ѧ������ʱ�����յ�����Ϊ___________kJ��
��2��CO��NO2��ԭΪ���ʵ��Ȼ�ѧ����ʽΪ_______��
��3��Ϊ��ģ�ⷴӦ2NO(g)��2CO(g) ![]() N2(g)��2CO2(g)�ڴ�ת�����ڵĹ������������һ���������÷�Ӧ�ں����ܱ������н��У��ô�������ò�ͬʱ��NO��CO��Ũ�����±���
N2(g)��2CO2(g)�ڴ�ת�����ڵĹ������������һ���������÷�Ӧ�ں����ܱ������н��У��ô�������ò�ͬʱ��NO��CO��Ũ�����±���
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
c(NO)/(10-4mol/L) | 10.0 | 4.50 | 2.50 | 1.50 | 1.00 | 1.00 |
c(CO)/(10-3mol/L) | 3.60 | 3.05 | 2.28 | 2.75 | 2.70 | 2.70 |
��ǰ2 s�ڵ�ƽ����Ӧ����v(N2)��___________,���¶��£��÷�Ӧ��ƽ�ⳣ��K��________��
����˵��������Ӧ�ﵽƽ��״̬����_________��
A��2n(CO2)��n(N2) B����������ƽ����Է�����������
C�������ܶȲ��� D������������ѹǿ����
����NO��COŨ�����ʱ����ϵ��NO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����NO��ƽ��ת�������¶����߶���С��ԭ����___________ ��ͼ��ѹǿ��p1��p2

