��Ŀ����
13���л���A�ɷ�����ͼת�������������ʾ�Ϊ�л����������������ȥ����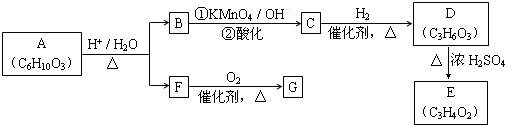
��֪��
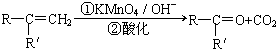 ��R��R��ɱ�ʾ����������ţ�
��R��R��ɱ�ʾ����������ţ���ش�
��1��F�����ܶ�����ͬ������H2�ܶȵ�31�����ҷ�����������֪1mol F�����������ò���H2 22.4L����״��������F�ķ���ʽ��C2H6O2��
��2��G��F����Է�������֮��Ϊ4����G��������ac������ĸ����
a������������Һ��Ӧ b���������ᷢ��������Ӧ
c���������������ӳɷ�Ӧ d��1mol G����2mol����Cu��OH��2������Ӧ
��3��D����NaHCO3��Ӧ����������D���Է�Ӧ�õ�������Ԫ����������E��ʹ������Ȼ�̼��Һ��ɫ����D��E�Ļ�ѧ����ʽ��CH3CH��OH��COOH$\frac{H_{2}SO_{4}��Ũ��}{��}$CH2�TCH-COOH+H2O��
��4��B��ͬ���칹��϶࣬д��һ�ֲ������ܷ���������Ӧ��ͬ���칹��ṹ��ʽ��HCOOCH2-CH=CH2��OHCCH2CH2CHO�ȣ�
��5��Aת��ΪB��F�Ļ�ѧ����ʽ��
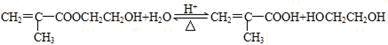 ��
����6��ij������H��̼ԭ����С��10���˴Ź�������ֻ��һ��壬����ͬ������Ҳ�ɷ�������B��C�ķ�Ӧ��ֻ����һ���л���I��I�Ľṹ��ʽ����CH3��2C=O��
���� F�������ܶ�����ͬ������H2�ܶȵ�31����������Է�������Ϊ62��1mol F���������������ò���H2 22.4L����״��������1molH2��˵��F�к���2��-OH��ӦΪ�Ҷ�������C2H6O2��������������ȩ������R-CH2OH��RCHO��֪��������Է����������2��G��F����Է�������֮��Ϊ4����GӦΪOHC-CHO��Ϊ�Ҷ�ȩ��A����B��F�ķ�ӦӦΪ����ˮ�⣬��B�ķ���ʽӦΪC4H6O2����������Ϣ��֪ӦΪ ��AΪ
��AΪ ��CΪ
��CΪ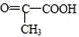 �������������ӳɷ�Ӧ����D��DӦΪCH3CH��OH��COOH��CH3CH��OH��COOH��Ũ���������·�����ȥ��Ӧ����E����EΪCH2=CHCOOH������л���Ľṹ�����ʽ����⣮
�������������ӳɷ�Ӧ����D��DӦΪCH3CH��OH��COOH��CH3CH��OH��COOH��Ũ���������·�����ȥ��Ӧ����E����EΪCH2=CHCOOH������л���Ľṹ�����ʽ����⣮
��� �⣺F�������ܶ�����ͬ������H2�ܶȵ�31����������Է�������Ϊ62��1mol F���������������ò���H2 22.4L����״��������1molH2��˵��F�к���2��-OH��ӦΪ�Ҷ�������C2H6O2��������������ȩ������R-CH2OH��RCHO��֪��������Է����������2��G��F����Է�������֮��Ϊ4����GӦΪOHC-CHO��Ϊ�Ҷ�ȩ��A����B��F�ķ�ӦӦΪ����ˮ�⣬��B�ķ���ʽӦΪC4H6O2����������Ϣ��֪ӦΪ ��AΪ
��AΪ ��CΪ
��CΪ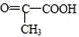 �������������ӳɷ�Ӧ����D��DӦΪCH3CH��OH��COOH��CH3CH��OH��COOH��Ũ���������·�����ȥ��Ӧ����E����EΪCH2=CHCOOH��
�������������ӳɷ�Ӧ����D��DӦΪCH3CH��OH��COOH��CH3CH��OH��COOH��Ũ���������·�����ȥ��Ӧ����E����EΪCH2=CHCOOH��
��1��F�������ܶ�����ͬ������H2�ܶȵ�31����������Է�������Ϊ62��1mol F���������������ò���H2 22.4L����״��������1molH2��˵��F�к���2��-OH��ӦΪ�Ҷ�������C2H6O2��
�ʴ�Ϊ��C2H6O2��
��2��GӦΪOHC-CHO��Ϊ�Ҷ�ȩ������-CHO���ɷ��������ͼӳɷ�Ӧ�����ܷ���������Ӧ������2��-CHO����1molG������4mol����Cu��OH��2������Ӧ��
�ʴ�Ϊ��ac��
��3��DӦΪCH3CH��OH��COOH��CH3CH��OH��COOH��Ũ���������·�����ȥ��Ӧ����E����EΪCH2=CHCOOH����Ӧ�ķ���ʽΪCH3CH��OH��COOH$\frac{H_{2}SO_{4}��Ũ��}{��}$CH2�TCH-COOH+H2O��
�ʴ�Ϊ��CH3CH��OH��COOH$\frac{H_{2}SO_{4}��Ũ��}{��}$CH2�TCH-COOH+H2O��
��4��BΪ ��B��ͬ���칹�岻�����ܷ���������Ӧ����ýṹ��ʽΪHCOOCH2-CH=CH2��OHCCH2CH2CHO�ȣ�
��B��ͬ���칹�岻�����ܷ���������Ӧ����ýṹ��ʽΪHCOOCH2-CH=CH2��OHCCH2CH2CHO�ȣ�
�ʴ�Ϊ��HCOOCH2-CH=CH2��OHCCH2CH2CHO�ȣ�
��5��AΪ ����ˮ������
����ˮ������ ���Ҷ�������Ӧ�ķ���ʽΪ
���Ҷ�������Ӧ�ķ���ʽΪ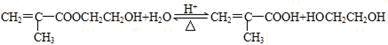 ��
��
�ʴ�Ϊ��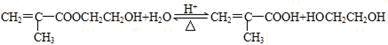 ��
��
��6��ij������H��̼ԭ����С��10���˴Ź�������ֻ��һ��壬����ͬ������Ҳ�ɷ�������B��C�ķ�Ӧ��ֻ����һ���л���I��������ĽṹΪ��CH3��2C=C��CH3��2������I�Ľṹ��ʽΪ��CH3��2C=O��
�ʴ�Ϊ����CH3��2C=O��
���� ���⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ������һ��Ҫץס�����Ϣ�����������ʵ������еĹ������жϿ��ܾ��е����ʣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | һ�� | B�� | ���� | C�� | ���� | D�� | ���� |
| A�� | �غ�ɫNO2��ѹ����ɫ�ȱ�����dz | |
| B�� | Fe��SCN��3��Һ�м������KSCN����ɫ���� | |
| C�� | ��ˮ�˱����ڵ��¡��ܹ������� | |
| D�� | SO2��������SO3�ķ�Ӧ��������������Ŀ��� | |
| E�� | ���������д�������ð�� | |
| F�� | �Ӵ�����ʹ������������һ��������ת��Ϊ���� |
| A�� | ��Ӧǰ����������ʵ���Ũ�Ȳ�ͬ | |
| B�� | ��Ӧ��ʼ�������H+Ũ�ȼ�С�� | |
| C�� | ��Ӧ��ʼ���������������������ͬ | |
| D�� | �ӷ�Ӧ��ʼ���������������õ�ʱ����� |
| A�� | ���������գ� | B�� | ���������ȣ� | ||
| C�� | �������������Һ | D�� | Ũ���� |