ЬтФПФкШн
ЁОЬтФПЁПЯТСаЫЕЗЈе§ШЗЕФЪЧЃКЃЈ ЃЉ
ЂйClЃЕФНсЙЙЪОвтЭМ
ЂкєЧЛљЕФЕчзгЪНЃК![]()
ЂлHClOЕФНсЙЙЪНЃКHЁЊClЁЊO
ЂмNaHCO3дкЫЎжаЕФЕчРыЗНГЬЪНЃКNaHCO3=NaЃЋЃЋHЃЋЃЋCO32-
ЂнNa2OЕФЫЎШмвКФмЕМЕчЃЌетВЛФмЫЕУїNa2OЪЧЕчНтжЪ
ЂоSiO2МШФмгыЧтЗњЫсЗДгІгжФмгыNaOHШмвКЗДгІЃЌЙЪSiO2ЪЧСНадбѕЛЏЮяЃЛ
ЂпЗжСѓЁЂИЩСѓЁЂСбЛЏЖМЪЧЛЏбЇБфЛЏЃЈ ЃЉ
A. ЂйЂкЂн B. ЂйЂмЂоЂп C. ЂкЂлЂмЂо D. ЂкЂлЂнЂоЂп
ЁОД№АИЁПA
ЁОНтЮіЁПЪдЬтЗжЮіЃКЂйТШРызгЕФКЫФкга17ИіжЪзгЃЌКЫЭтга18ИіЕчзгЃЌе§ШЗЃЛЂкєЧЛљЕФЕчзгЪН![]() ЃЌе§ШЗЃЛЂлHClOЕФНсЙЙЪНЃКHЁЊOЁЊClЃЌДэЮѓЃЛЂмNaHCO3дкЫЎжаЕФЕчРыЗНГЬЪНЃКNaHCO3ЃНNaЃЋЃЋHCO3-ЃЌДэЮѓЃЛЂнNa2OЕФЫЎШмвКФмЕМЕчЃЌетВЛФмЫЕУїNa2OЪЧЕчНтжЪЃЌжЛгаШлШкЕФNa2OЕМЕчВХПЩвдЫЕУїЃЌе§ШЗЃЛЂоSiO2МШФмгыЧтЗњЫсЗДгІгжФмгыNaOHШмвКЗДгІЃЌЕЋSiO2ВЛЪЧСНадбѕЛЏЮяЃЌДэЮѓЃЛЂпЗжСѓЪЧЮяРэБфЛЏЃЌДэЮѓЃЌД№АИбЁAЁЃ
ЃЌе§ШЗЃЛЂлHClOЕФНсЙЙЪНЃКHЁЊOЁЊClЃЌДэЮѓЃЛЂмNaHCO3дкЫЎжаЕФЕчРыЗНГЬЪНЃКNaHCO3ЃНNaЃЋЃЋHCO3-ЃЌДэЮѓЃЛЂнNa2OЕФЫЎШмвКФмЕМЕчЃЌетВЛФмЫЕУїNa2OЪЧЕчНтжЪЃЌжЛгаШлШкЕФNa2OЕМЕчВХПЩвдЫЕУїЃЌе§ШЗЃЛЂоSiO2МШФмгыЧтЗњЫсЗДгІгжФмгыNaOHШмвКЗДгІЃЌЕЋSiO2ВЛЪЧСНадбѕЛЏЮяЃЌДэЮѓЃЛЂпЗжСѓЪЧЮяРэБфЛЏЃЌДэЮѓЃЌД№АИбЁAЁЃ

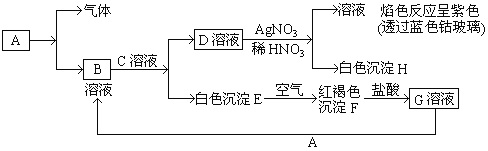
 бєЙтЪдОэЕЅдЊВтЪдОэЯЕСаД№АИ
бєЙтЪдОэЕЅдЊВтЪдОэЯЕСаД№АИЁОЬтФПЁПИљОнЯТСаЪЕбщВйзїКЭЯжЯѓЫљЕУГіЕФНсТле§ШЗЕФЪЧ
бЁЯю | ЪЕбщВйзїКЭЯжЯѓ | НсТл |
A | ЯђБНЗгзЧвКжаЕЮМгNa2CO3ШмвКЃЌзЧвКБфЧх | БНЗгЕФЫсадЧПгкH2CO3ЕФЫсад |
B | ЯђЕтЫЎжаМгШыЕШЬхЛ§CCl4ЃЌеёЕДКѓОВжУЃЌЩЯВуНгНќЮоЩЋЃЌЯТВуЯдзЯКьЩЋ | I2дкCCl4жаЕФШмНтЖШДѓгкдкЫЎжаЕФШмНтЖШ |
C | ЯђCuSO4ШмвКжаМгШыЬњЗлЃЌгаКьЩЋЙЬЬхЮіГі | Fe2+ЕФбѕЛЏадЧПгкCu2+ЕФбѕЛЏад |
D | ЯђNaClЁЂNaIЕФЛьКЯЯЁШмвКжаЕЮШыЩйСПЯЁAgNO3ШмвКЃЌгаЛЦЩЋГСЕэЩњГЩ | Ksp(AgCl) >Ksp(AgI) |
A. A B. B C. C D. D
ЁОЬтФПЁПЯжгаВПЗждЊЫиЕФдзгНсЙЙЬиЕуШчБэЃК
X | LВуЕчзгЪ§ЪЧKВуЕчзгЪ§ЕФ3БЖ |
Y | КЫЭтЕчзгВуЪ§ЕШгкдзгађЪ§ |
Z | LВуЕчзгЪ§ЪЧKВуКЭMВуЕчзгЪ§жЎКЭ |
W | зюЭтВуЕчзгЪ§ЪЧДЮЭтВуЕчзгЪ§ЕФ2.5БЖ |
ЃЈ1ЃЉЛГіWдзгНсЙЙЪОвтЭМ________________________ЁЃ
ЃЈ2ЃЉдЊЫиXгыдЊЫиZЯрБШЃЌЗЧН№ЪєадНЯЧПЕФЪЧ________(ЬюдЊЫиУћГЦ)ЃЌаДГівЛИіФмБэЪОXЁЂZЗЧН№ЪєадЧПШѕЙиЯЕЕФЛЏбЇЗДгІЗНГЬЪНЃК________________________________________ЁЃ
ЃЈ3ЃЉXЁЂYЁЂZЁЂWЫФжждЊЫиаЮГЩЕФвЛжжРызгЛЏКЯЮяЃЌЦфЫЎШмвКЯдЧПЫсадЃЌИУЛЏКЯЮяЕФЛЏбЇЪНЮЊ____________________ЁЃ
ЃЈ4ЃЉдЊЫиXКЭдЊЫиYвддзгИіЪ§БШ1ЁУ1ЛЏКЯаЮГЩЕФЛЏКЯЮяQЃЌаДГіQЕФЕчзгЪН________ЁЃдЊЫиWКЭдЊЫиYЛЏКЯаЮГЩЕФЛЏКЯЮяMЃЌQКЭMЕФЕчзгзмЪ§ЯрЕШЁЃвдMЮЊШМСЯЃЌQЮЊбѕЛЏМСЃЌПЩзїЛ№М§ЭЦНјМСЃЌзюжеЩњГЩЮоЖОЕФЁЂЧвдкздШЛНчжаЮШЖЈДцдкЕФЮяжЪЃЌаДГіИУЗДгІЕФЛЏбЇЗНГЬЪНЃК____________________________________ЁЃ
ЁОЬтФПЁПЕЅОЇЙшЪЧаХЯЂВњвЕжаживЊЕФЛљДЁВФСЯЁЃЭЈГЃгУЬМдкИпЮТЯТЛЙдЖўбѕЛЏЙшжЦЕУДжЙшЃЈКЌЬњЁЂТСЁЂХ№ЁЂСзЕШдгжЪЃЉЃЌДжЙшгыТШЦјЗДгІЩњГЩЫФТШЛЏЙшЃЈЗДгІЮТЖШ450-500ЁцЃЉЃЌЫФТШЛЏЙшОЬсДПКѓгУЧтЦјЛЙдПЩЕУИпДПЙшЁЃвдЯТЪЧЪЕбщЪвжЦБИЫФТШЛЏЙшЕФзАжУЪОвтЭМЁЃ
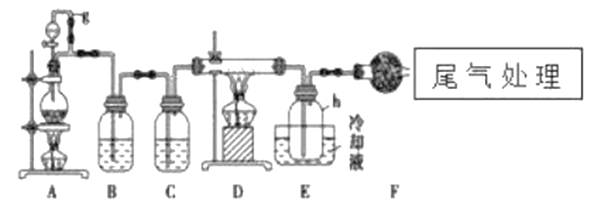
ЯрЙиаХЯЂШчЯТЃК
a.ЫФТШЛЏЙшНгДЅЫЎЛсЗЂЩњЛЏбЇЗДгІЃЛ
b.Х№ЁЂТСЁЂЬњЁЂСздкИпЮТЯТОљФмгыТШЦјжБНгЗДгІЩњГЩЯргІЕФТШЛЏЮяЃЛ
c.гаЙиЮяжЪЕФЮяРэГЃЪ§МћЯТБэЃК
ЮяжЪ | SiCl4 | BC13 | A1C13 | FeCl3 | PCl5 |
ЗаЕу/Ёц | 57.7 | 12.8 | Ѓ | 315 | Ѓ |
ШлЕу/Ёц | -70.0 | -107.2 | Ѓ | Ѓ | Ѓ |
Щ§ЛЊЮТЖШ/Ёц | Ѓ | Ѓ | 180 | 300 | 162 |
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉаДГізАжУAжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЃК___________ЃЌDжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪН___________ЁЃ
ЃЈ2ЃЉAжаgЙмЕФзїгУЪЧ__________________ЃЌзАжУCжаЕФЪдМСЪЧ___________ЃЌзїгУЪЧ___________ЁЃ
ЃЈ3ЃЉзАжУEжаЕФhЦПашвЊРфШДЕФРэгЩЪЧ______________________ЁЃ
ЃЈ4ЃЉзАжУEжаhЦПЪеМЏЕНЕФДжВњЮяПЩЭЈЙ§ОЋСѓЃЈРрЫЦЖрДЮеєСѓЃЉЕУЕНИпДПЖШЫФТШЛЏЙшЃЌОЋСѓКѓЕФВаСєЮяжаЃЌГ§ЬњдЊЫиЭтПЩФмЛЙКЌгаЕФдгжЪдЊЫиЪЧ___________ЃЈЬюаДдЊЫиЗћКХЃЉЁЃ
ЃЈ5ЃЉЙ§СПЕФТШЦјПЩвдгУЪЏЛвШщРДДІРэЃЌЧыаДГіИУЗДгІЕФЛЏбЇЗНГЬЪН_____________________ЁЃ