��Ŀ����
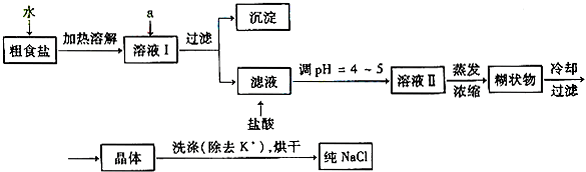
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϣ���1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO42-���������ӣ�ʵ�����ᴿNaCl��������ͼ1��

�ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba��NO3��2��Һ�����Ȼ�̼
��1��������ȥ��Һ���е�Ca2+��Mg2+��Fe3+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ
��Ϊ�˼���NaCl��������Ƿ���KCl������ѡ��ķ���Ϊ
��2�����ᴿ��NaCl����500mL4.00mol?L-1NaCl��Һ������������ҩ�ס���������еIJ�������
��3����ⱥ��ʳ��ˮ��װ����ͼ2��ʾ����һ���������£����ռ���H2Ϊ2L����ͬ���������ռ���Cl2
��4��ʵ�����Ʊ�H2��Cl2ͨ���������з�Ӧ��Zn+H2SO4��ϡ���TZnSO4+H2����MnO2+4HCl��Ũ��
| ||

��������1���ٰ�����ת��Ϊ�����������ȥ������������̼������ӣ��������ӡ�þ���������������ӣ�������������ñ����ӣ�Ҫע������ʵ�˳��ӵ��Լ����ܰ�ǰ���ȼ��ǹ����Լ��������ڼ���ϴ��Һ���Ƿ��м����ӣ�
��2������������Һ�IJ������ѡ������������
��3�����ݵ�ⱥ��ʳ��ˮʱ�IJ����Լ��������������Ƶķ�Ӧ���ش�
��4��ʵ�����в��ý���п���ᷴӦ��������������ö������̺�Ũ�������������������ݴ˽��װ���ص���
��2������������Һ�IJ������ѡ������������
��3�����ݵ�ⱥ��ʳ��ˮʱ�IJ����Լ��������������Ƶķ�Ӧ���ش�
��4��ʵ�����в��ý���п���ᷴӦ��������������ö������̺�Ũ�������������������ݴ˽��װ���ص���
����⣺��1���ٳ�ȥ�����еĿ��������ʣ�Ca2+��Mg2+��Fe3+��SO42-����ʱ�����Լ������NaOH��ȥ��þ���Ӻ������ӣ���Mg2++2OH-=Mg��OH��2����Fe3++3OH-�TFe��OH��3�����������BaCl2��ȥ����������ӣ���SO42-+Ba2+=BaSO4�����������Na2CO3��ȥ�������ӵĶ���ı����ӣ���Ca2++CO32-=CaCO3����̼���Ʊ�������Ȼ���֮���������Ba2+���������ƺ��Ȼ������Եߵ������˳��
������μ�˳����BaCl2��NaOH��NaCO3������NaOH��BaCl2��Na2CO3����
�ʴ�Ϊ��BaCl2��NaOH��Na2CO3����NaOH��BaCl2��Na2CO3����
�ڼ���ϴ��Һ���Ƿ��м����ӣ����øɾ���˿ȡ���һ��ϴ��Һ�ھƾ��������գ�����ɫ�ܲ������粻���ֵ���ɫ���棬��˵����ϴ����
�ʴ�Ϊ������ɫ�ܲ����۲��ɫΪ����ɫ֤�����Ȼ��ش��ڣ�
��2������500mL4.00mol?L-1NaCl��Һ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬�ò��������裬�����ܽ⣬�ָ����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������Ҫ��������������ƽ���ձ�����������500ml������ƿ����ͷ�ιܡ�ҩ�ף�ʹ�û���Ҫ�ձ���500ml������ƿ����ͷ�ιܣ�
�ʴ�Ϊ���ձ���500mL����ƿ����ͷ�ιܣ�
��3����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O
2NaOH+Cl2��+H2�������������ڲ�����Cl2����������ɵ�NaOH��ӦNaCl��NaClO��H2O��ʹ�ò��ֵ�Cl2�����ģ�����ͬ���������ռ�����Cl2С��2L��
�ʴ�Ϊ������������ɵ������������ɵ�NaOH�����˷�Ӧ��
��4��ʵ�����в��ý���п������ȷ�Ӧ�������������ѡ��װ��e�����ö������̺�Ũ���������������������е������Ȼ�����Լӱ���ʳ��ˮ����������ˮ���Բ���Ũ��������ȥ���������ſ������ռ�������ѡ��װ��d��
�ʴ�Ϊ��e��d
������μ�˳����BaCl2��NaOH��NaCO3������NaOH��BaCl2��Na2CO3����
�ʴ�Ϊ��BaCl2��NaOH��Na2CO3����NaOH��BaCl2��Na2CO3����
�ڼ���ϴ��Һ���Ƿ��м����ӣ����øɾ���˿ȡ���һ��ϴ��Һ�ھƾ��������գ�����ɫ�ܲ������粻���ֵ���ɫ���棬��˵����ϴ����
�ʴ�Ϊ������ɫ�ܲ����۲��ɫΪ����ɫ֤�����Ȼ��ش��ڣ�
��2������500mL4.00mol?L-1NaCl��Һ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬�ò��������裬�����ܽ⣬�ָ����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������Ҫ��������������ƽ���ձ�����������500ml������ƿ����ͷ�ιܡ�ҩ�ף�ʹ�û���Ҫ�ձ���500ml������ƿ����ͷ�ιܣ�
�ʴ�Ϊ���ձ���500mL����ƿ����ͷ�ιܣ�
��3����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O
| ||
�ʴ�Ϊ������������ɵ������������ɵ�NaOH�����˷�Ӧ��
��4��ʵ�����в��ý���п������ȷ�Ӧ�������������ѡ��װ��e�����ö������̺�Ũ���������������������е������Ȼ�����Լӱ���ʳ��ˮ����������ˮ���Բ���Ũ��������ȥ���������ſ������ռ�������ѡ��װ��d��
�ʴ�Ϊ��e��d
������������Ҫ�����˴����ᴿ�����еij��ӡ���Һ�����ơ����ʳ��ˮ�ȷ����֪ʶ���ۺ���ǿ��ע������ʵ�˳��Ҫ�����µ����ʣ�������Һ�����Ʒ����͵��ԭ���ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ