��Ŀ����
����Ŀ��ij�¶�ʱ����һ��2L���ܱ������У�X��Y��Z�������ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף�

��1���÷�Ӧ�Ļ�ѧ����ʽΪ_________
��2����Ӧ��ʼ��2min��������Z��ʾ��ƽ����Ӧ����Ϊ_________��
��3����X��Y��Z��Ϊ���壬2min��Ӧ�ﵽƽ�⣬��Ӧ��ƽ��ʱ��
�ٴ�ʱ��ϵ��ѹǿ�ǿ�ʼʱ��______����
�ڴ�ƽ��ʱ�������ڻ�������ƽ����Է�����������ʼͶ��ʱ________���������С������ȡ�����
��4���˷�Ӧ��ƽ�����ֻ�Ӵ���ϵѹǿ��Z�����ʵ������٣���Y�����壬��X��״̬��_______��
���𰸡� 3X+Y![]() 2Z 0.05mol/��L��min�� 0.9 ���� �������Һ��
2Z 0.05mol/��L��min�� 0.9 ���� �������Һ��
����������1������ͼ���֪����Ӧ���е�2minʱ���ʵ�Ũ�Ȳ��ٷ����仯��˵����Ӧ�ﵽƽ��״̬����ʱ����X��Y�����ʵ����ֱ���0.3mol��0.1mol������Z�����ʵ�����0.2mol�����Ը��ݱ仯��֮������Ӧ�Ļ�ѧ������֮�ȿ�֪���÷�Ӧ�Ļ�ѧ����ʽ��3X��Y![]() 2Z��
2Z��
��2���ӿ�ʼ��2min��Z��ƽ����Ӧ����Ϊ![]() ��0.05mol/(L��min)��
��0.05mol/(L��min)��
��3�������¶Ȳ���ʱ�������ѹǿ�������������ʵ��������ȣ���Ӧǰ��������ʵ���Ϊ2mol����Ӧ��ʼ��2min������ĵ����ʵ���Ϊ1.8mol����ʱ��ϵ��ѹǿ�ǿ�ʼʱ��0.9�����ڷ�Ӧǰ���������������䣬����������ʵ����ڼ��٣�ƽ��ʱ�������ڻ�������ƽ����Է�������������
��4���˷�Ӧ��ƽ�����ֻ�Ӵ���ϵѹǿ��ʹZ�����ʵ������٣�ƽ�������ƶ�����XӦ���Ƿ���̬������

����Ŀ���״�������Ҫ�Ļ���ԭ�ϣ��ֿ���Ϊȼ�ϣ���������Ϊȼ�ϵ�ص�ԭ�ϡ����úϳ���(��Ҫ�ɷ�ΪCO��CO2��H2)�ڴ����������ºϳɼ״�����������Ҫ��Ӧ����:
��CO(g)+2H2(g) ![]() CH3OH(g) ��H1 ����֪��CO�Ľṹ��N2���ƣ�
CH3OH(g) ��H1 ����֪��CO�Ľṹ��N2���ƣ�
��CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g)��H2
CH3OH(g)+H2O(g)��H2
��CO2(g)+H2(g) ![]() CO(g)+H2O(g) ��H3
CO(g)+H2O(g) ��H3
�ش���������:
(1)��֪��Ӧ������صĻ�ѧ��������������:
��ѧ�� | H-H | C-O | C=O | H-O | C-H |
E/(KJ/mol) | 436 | 343 | 1076 | 465 | 413 |
�ɴ˼�����H1=______kJ��mol-1����֪��H2=-58kJ��mol-1������H3=_____kJ��mol-1��
(2)��֪:
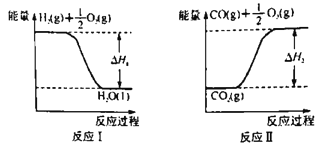
���״���ȼ����Ϊ��H3��������H1����H2����H3��ʾCO(g)+2H2(g) ![]() CH3OH(l)����H=________������H1=-285.8 kJ��mol-1����H2=+283.0 kJ��mol-1��ijH2��CO�Ļ��������ȫȼ��ʱ�ų�113.74 kJ������ͬʱ����3.6gҺ̬ˮ����ԭ���������H2��CO�����ʵ���֮��Ϊ______��
CH3OH(l)����H=________������H1=-285.8 kJ��mol-1����H2=+283.0 kJ��mol-1��ijH2��CO�Ļ��������ȫȼ��ʱ�ų�113.74 kJ������ͬʱ����3.6gҺ̬ˮ����ԭ���������H2��CO�����ʵ���֮��Ϊ______��
(3)��CH4��H2OΪԭ�ϣ�ͨ�����з�ӦҲ�����Ʊ��״���
��:CH4(g)+H2O(g)=CO(g)+3H2(g) ��H=+206.0 kJ��mol-1
��:CO(g)+2H2(g)= =CH3OH (g)��H=-129.0 kJ��mol-1
CH4(g)+H2O(g)��Ӧ����CH3OH(g)��H2(g)���Ȼ�ѧ����ʽΪ__________.
