��Ŀ����
����Ŀ����ͼΪʵ������ȡ�����������Cl2�������м���Cl2���ʵ�ʵ��װ�á�����Dƿ�з��и���ĺ�ɫ������E��Ϊͭ����E�Ҷ˳����ܿڸ���Ϊ�����Իش�
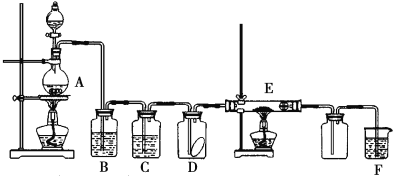
��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ____���������������ڱ�״���µ����Ϊ22.4L����������HCl������Ϊ____��
��2��Ϊ�õ����﴿����������һ��B��ʢ�ŵ��Լ�Ϊ____�� C��ʢ�ŵ��Լ�Ϊ____��
��3��E����������Ӧ�Ļ�ѧ����ʽΪ____��
��4��Fװ�õ�������____���䷴Ӧ�Ļ�ѧ����ʽΪ____��
���𰸡�4HCl(Ũ)��MnO2![]() MnCl2��2H2O��Cl2�� 73g ����ʳ��ˮ Ũ���� Cu+Cl2
MnCl2��2H2O��Cl2�� 73g ����ʳ��ˮ Ũ���� Cu+Cl2![]() CuCl2 �������� Cl2+2NaOH=NaCl+NaClO+H2O
CuCl2 �������� Cl2+2NaOH=NaCl+NaClO+H2O
��������
��1��ʵ���������ö������̺�Ũ������ȷ�Ӧ��������������A����Ũ���ᣬB���Ƕ������̹��壬��Ӧ�Ļ�ѧ����ʽΪ4HCl(Ũ)��MnO2![]() MnCl2��2H2O��Cl2�����ڸ÷�Ӧ�У������ڱ�״���µ����Ϊ22.4L�����ʵ���Ϊ1mol��HCl����������Cl2����������HCl�����ʵ���Ϊ2mol������Ϊ73g��
MnCl2��2H2O��Cl2�����ڸ÷�Ӧ�У������ڱ�״���µ����Ϊ22.4L�����ʵ���Ϊ1mol��HCl����������Cl2����������HCl�����ʵ���Ϊ2mol������Ϊ73g��
��2�����ɵ������к����Ȼ����ˮ�������ʣ��ñ���ʳ��ˮ��ȥ�����е��Ȼ��⣬����B���Լ�Ϊ����ʳ��ˮ����Ũ�����ȥ�����е�ˮ�����õ����﴿��������������C���Լ�������Ϊ���������е�ˮ�������ʴ�Ϊ������ʳ��ˮ��Ũ���
��3��E����ͭ�������Ⱥ�������Ӧ�����Ȼ�ͭ�����ɵ��Ȼ�ͭ������ʽΪ��Cu+Cl2![]() CuCl2��
CuCl2��
��4�������ж��������ŷŵ������У���Ҫ������������Һ���գ�Fװ����������������������������ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��