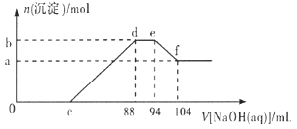
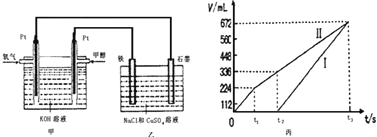
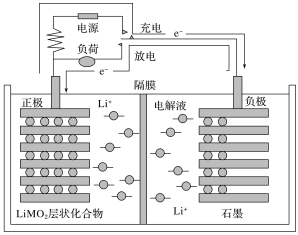
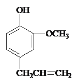
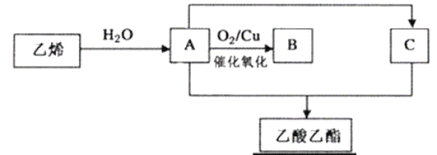
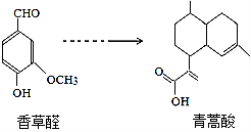
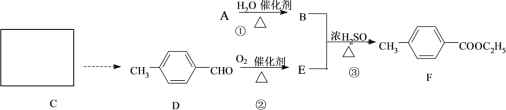��Ŀ����
����Ŀ��Fe��Co��Ni��Ϊ�ڢ���Ԫ�أ����ǵĻ��������������������Ź㷺��Ӧ�á�
��1����̬Coԭ�ӵļ۵����Ų�ʽΪ____________
��2����֪HN3��һ�����ᣬ����ˮ��Һ�еĵ��뷽��ʽΪHN3![]() H++N3-,��N3-��Ϊ�ȵ������һ�ַ���Ϊ��_______��N3-�����ӻ�����Ϊ___________��
H++N3-,��N3-��Ϊ�ȵ������һ�ַ���Ϊ��_______��N3-�����ӻ�����Ϊ___________��
��3��Co3+��һ��������[Co(N3)(NH3)5]2+�У�Co3+ ����λ����___________��1mol����������������������ĿΪ____����λ��NH3�Ŀռ乹��Ϊ��___________ ��
��4��ij��ɫ�����У�Fe2+��Fe3+�ֱ�ռ�������廥�����ڵĶ��㣬���������ÿ�����Ͼ���һ��CN-��K+λ���������ijǡ��λ���ϡ��ݴ˿�֪�þ���Ļ�ѧʽΪ____________����������Fe2+�����������γɵĿռ乹����_____________��
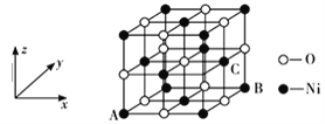
��5��NiO�ľ���ṹ����ͼ��ʾ�����������������AΪ(0��0��0)��BΪ(1��1��0)����C�����������Ϊ_______________��
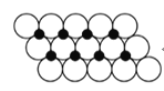
��6��һ���¶��£�NiO��������Է��ط�ɢ���γ��������Ӳ㡱��������ΪO2-�����õ������У�Ni2+������У�����ͼ������֪O2-�İ뾶Ϊa pm��ÿƽ��������Ϸ�ɢ�ĸþ��������Ϊ__________g���ú�a��NA�Ĵ���ʽ��ʾ����
���𰸡�3d74s2 CO2 sp 6 23NA ������ KFe2(CN)6 ���������� (1��1/2��1/2) ![]() ����
����![]() ��
��
��������
�ڣ�2���ʣ�ԭ������ͬ������������ͬ�ķ���,����Ϊ�ȵ����壻�ڣ�6���ʣ���ÿƽ��������Ϸ�ɢ�ĸþ��������������ͼ�м��ι�ϵ���ÿ����������ռ��������������ÿƽ�����е�����������������ÿ������������������ÿƽ�����е���������������ע�ⵥλ�Ļ��㡣
��1��COԭ�ӵĺ˵����Ϊ27����̬Coԭ�ӵļ۵����Ų�ʽΪ3d74s2��
��2����N3-��Ϊ�ȵ������һ�ַ���Ϊ��CO2���ӻ������=����ԭ�ӵŵ��Ӷ���+����ԭ�ӵ���������Ŀ������N3-���ĵ�ԭ�ӹµ��Ӷ���Ϊ0����������ĿΪ2�������ӻ������Ϊ2����N3-�����ӻ�����Ϊsp��
��3��Co3+��һ��������[Co(N3)(NH3)5]2+�У��������Ӻ�N3-�е�ԭ�����йµ��Ӷԣ��ܹ���Co+�γ���λ��������5�������Ӻ�1��N3-���ӣ�Co3+����λ����6��5 mol���������ṩ����Ϊ15mol��1 mol N3-�к�������2 mol���γ���λ����6 mol����1mol����������������������ĿΪ23NA����λ��NH3�Ŀռ乹��Ϊ�������Σ�
��4��Fe2+��Fe3+ռ��������Ļ������ڵĶ��㣬��ÿ������������4��Fe2+��4��Fe3+�����ݾ���Ŀռ�ṹ�ص㣬ÿ�������ϵ�������1/8���ڸ������壬�������������1/2��Fe2+��1/2��Fe3+��CN-λ������������ϣ����ϵ�����1/4���ڸ������壬������������3��CN-�����Ըþ���Ļ�ѧʽΪ [FeFe(CN)6]-���������ʳʵ����ԣ�������Ҫһ����������֮��ϣ����Ըþ���Ļ�ѧʽΪKFe2(CN)6����������Fe2+�����������γɵĿռ乹�������������Σ�
��5��NiO�ľ����������������AΪ(0��0��0)��BΪ(1��1��0)������ͼ�ɿ���C����������xΪ1����yΪ1/2����zΪ1/2����C�����������Ϊ(1��1/2��1/2)��
��6�����ݽṹ֪�������Ӻ����ڵ�������֮��ľ���Ϊ2a��������������������Ӻ˼�ľ����Ǿ�������������Ӻ������Ӿ����![]() ���������������2
���������������2![]() am������ͼƬ֪��ÿ����������ռ�����=2a m��2a m��sin60���10-24����ÿƽ�����е�����������=1/(2a m��2a m��sin60���10-24)=
am������ͼƬ֪��ÿ����������ռ�����=2a m��2a m��sin60���10-24����ÿƽ�����е�����������=1/(2a m��2a m��sin60���10-24)= ![]() ��1024��ÿ��������������=
��1024��ÿ��������������= ![]() g������ÿƽ�����е�����������=
g������ÿƽ�����е�����������=![]() ����
����![]() ��g��
��g��

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�