��Ŀ����
Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
(1)ʵ���ã�1 g�״��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�22.7 kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ__________________________________
(2)��֪��ӦCH3��CH3(g)�D��CH2=CH2(g)��H2(g)���йػ�ѧ���ļ������¡�
| ��ѧ�� | C��H | C=C | C��C | H��H |
| ����/kJ��mol��1 | 414.4 | 615.3 | 347.4 | 435.3 |
�Լ���÷�Ӧ�ķ�Ӧ��___________________________
(3)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�����������㡣�����������Ȼ�ѧ����ʽ�����㷴Ӧ2C(s)��2H2(g)��O2(g)=CH3COOH(l)���ʱ䦤H��________��
��CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��1/2O2(g)=H2O(l)
��H3����285.8 kJ��mol��1
(1)2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(l)
����H����1 452.8 kJ��mol��1
(2)��125.6 kJ��mol��1
(3)��488.3 kJ��mol��1
����

���ĺϳ�������Ҫ�Ļ�������֮һ��
I����ҵ�Ϻϳɰ��õ�H2�ж�����ȡ�ķ�����
�� �ý�̿��ˮ��Ӧ�� C(s��+ H2O(g) CO(g��+ H2(g)��
CO(g��+ H2(g)��
�� ����Ȼ����ˮ������Ӧ��CH4(g��+ H2O(g�� CO (g)+ 3H2(g)
CO (g)+ 3H2(g)
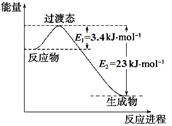
��֪�йط�Ӧ�������仯����ͼ�������з�Ӧ�Ħ�H =__________ ___��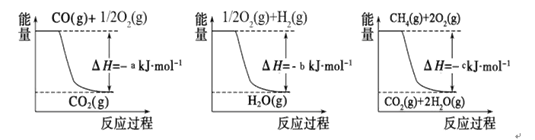
����3��1L���ܱ������У�ͬ�¶��¡�ʹ����ͬ�����ֱ���з�Ӧ��
3H2(g��+ N2(g�� 2NH3(g)������ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���Ӧ�ﵽƽ��ʱ�й�����Ϊ��
2NH3(g)������ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���Ӧ�ﵽƽ��ʱ�й�����Ϊ��
| �� �� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 3 mol H2��2 mol N2 | 6 mol H2��4mol N2 | 2 mol NH3 |
| �ﵽƽ���ʱ�䣨min�� | t | 5 | 8 |
| ƽ��ʱN2��Ũ�ȣ�mol��L-1�� | c1 | 3 | |
| N2��������� | ��1 | ��2 | ��3 |
| ��������ܶȣ�g��L-1�� | ��1 | ��2 | |
(1��������˵���÷�Ӧ�Ѵﵽƽ��״̬����
a��������N2��H2��NH3��Ũ��֮��Ϊ1�U3�U2
b��v��N2������3v��H2����
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���
(2���������дﵽƽ������Ҫ��ʱ��t 5min (��>��< ��=)
(3�����дӷ�Ӧ��ʼ��ƽ��ʱN2��ƽ����Ӧ���� ��ע����λ����
(4�������ϱ����ݣ����й�ϵ��ȷ����________��
a��2c1 =3mol/L b����1 = ��2 c��2��1 = ��2
(5�����¶��£��������У��÷�Ӧ��ƽ�ⳣ��K=____ __���÷�����ʾ����mol/L����2��
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
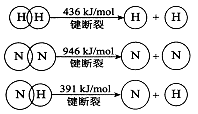
��1����֪��N2(g)+O2(g) = 2NO(g) ��H=+180��5kJ/mol
N2(g)+3H2(g)  2NH3(g) ��H=��92��4kJ/mol
2NH3(g) ��H=��92��4kJ/mol
2H2(g)+O2(g) = 2H2O(g) ��H=��483��6kJ/mol
д����������������ȫ����һ�����������ˮ�������Ȼ�ѧ����ʽΪ
��
��2��ij����С���о����������������������£��ı���ʼ�����������ʵ�����N2(g)+3H2(g) 2NH3(g)��Ӧ��Ӱ�졣
2NH3(g)��Ӧ��Ӱ�졣
ʵ������ͼ��ʾ��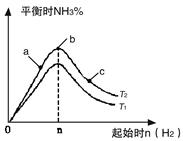
��ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�����
��ͼ����T2��T1�Ĺ�ϵ�ǣ�T2 T1������ڡ������ڡ������ڡ�����ȷ������
�ڱȽ���a��b��c����������ƽ��״̬�У���Ӧ��N2 ��ת������ߵ��ǣ�����ĸ���� ��
���������ݻ�Ϊ1L������ʼ��ϵ�м���1mol N2 ��n=3mol��Ӧ�ﵽƽ��ʱH2��ת����Ϊ60%����� �����£�T2������Ӧ��ƽ�ⳣ��K= ����������������䣬���������м���1mol N2��3mol H2��Ӧ�ﵽƽ��ʱ��������ת���ʽ�
����������������䡱����
��3��N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��
��һ���¶��£��ں����ܱ�������N2O5�ɷ������з�Ӧ��
2N2O5(g)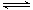 4NO2(g)��O2(g) ��H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ������
4NO2(g)��O2(g) ��H��0�±�Ϊ��Ӧ��T1�¶��µIJ���ʵ������
| t/s | 0 | 50 | 100 |
| c(N2O5)/mol��L��1 | 5��0 | 3��5 | 2��4 |
��50s��NO2��ƽ����������Ϊ ��
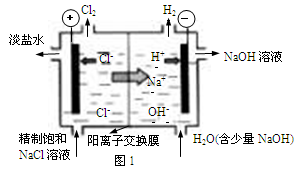
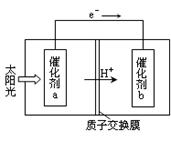
������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N2O5��װ����ͼ��ʾ������YΪCO2��
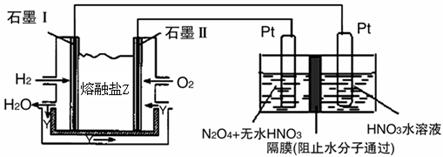
д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ ��
�ڵ���������N2O5�ĵ缫��ӦʽΪ ��
��֪��ѧ����������ʽ���ܿ����ת������д�±��Ŀհ�:
| ��ѧ��Ӧ����ʽ(����) | ����ת����ʽ |
| �� | �ɻ�ѧ��ת��Ϊ���� |
��Pb+PbO2+2H2SO4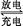 2PbSO4+2H2O 2PbSO4+2H2O | |
��CaCO3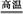 CaO+CO2�� CaO+CO2�� | |
������Ӧ������������ԭ��Ӧ����(�����)����
�п�Ժ�����о����о�Ա���ʽ���������ͬ�к������Ա�������PM2.5��ѧ��ɼ���Դ�ļ��ڱ仯�о����֣�����PM2.5��6����Ҫ��Դ�����У�����β����ȼú�ֱ�ռ4%��18%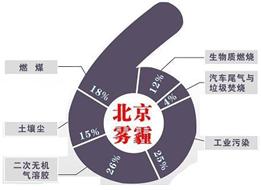
��1�����ھ�������β���ķ�ӦΪ��2NO(g)+2CO(g)
 2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
| A��װ��β������װ�õ������ų��������в��ٺ���NO��CO |
| B�����β������Ч�ʵij��÷����������¶� |
| C������ѹǿ������ƽ�����ƣ���ʵ�ʲ����п�ͨ����ѹ�ķ�ʽ����侻��Ч�� |
| D�����β������Ч�ʵ����;����ʹ�ø�Ч���� |

 Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ
Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ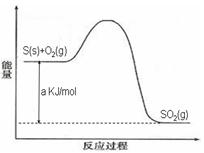
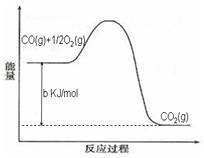
����SO2��ȥCO���Ȼ�ѧ����ʽΪ _____________________________________��
��3��NH3����ԭ�������SCR��������ĿǰӦ����㷺���������������ѳ������������Ļ�ѧ��Ӧ�ǣ�2NH3(g)+NO(g)+NO2(g)
 2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________��
2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________����4������ClO2�����������ﷴӦ�������£�

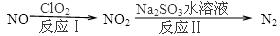
��Ӧ��Ļ�ѧ����ʽ��2NO+ClO2+H2O�TNO2+HNO3+2HCl����Ӧ������ӷ���ʽ�� ________________������11.2L N2���ɣ���״������������NO _________________ g��
��5����ҵ�����к��е�NO2�����õ�ⷨ��������NO2Ϊԭ�Ͽ���������ɫ������N2O5���Ʊ�����֮һ���Ƚ�NO2ת��ΪN2O4��Ȼ����õ�ⷨ�Ʊ� N2O5��װ����ͼ��ʾ�� Pt��Ϊ _____��������������N2O5�ĵ缫��Ӧʽ��________________��
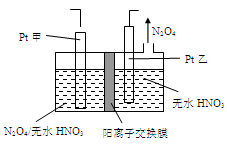
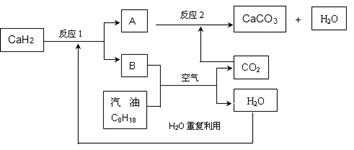
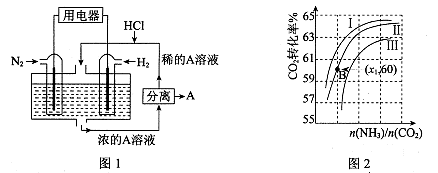
 CO(NH2)2��l��+ H2O(g)����ҵ����ʱ��ԭ��������ˮ������ͼ2��ʾCO2��ת�����백̼��
CO(NH2)2��l��+ H2O(g)����ҵ����ʱ��ԭ��������ˮ������ͼ2��ʾCO2��ת�����백̼��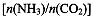 ��ˮ̼��
��ˮ̼��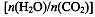 �ı仯��ϵ��
�ı仯��ϵ��
 Fe3����3H2O��ƽ�ⳣ��K�� ��
Fe3����3H2O��ƽ�ⳣ��K�� ��