��Ŀ����
��100��ʱ����0.100mol��N2O4�������1L��յ��ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ������
����գ�
��1���ﵽƽ��ʱN2O4��ת����Ϊ
��2��20s��������������Ũ��c1=
��3��������ͬ���������������������Ƕ�����������Ҫ�ﵽ����ͬ����ƽ��״̬��������������ʼŨ����
| ʱ��/s Ũ��mol?L-1 |
0 | 20 | 40 | 60 | 80 | 100 |
| c��N2O4��mol?L-1 | 0.100 | c1 | 0.050 | c3 | a | b |
| c��NO2��/mol?L-1 | 0.000 | 0.060 | c2 | 0.120 | 0.120 | 0.120 |
��1���ﵽƽ��ʱN2O4��ת����Ϊ
60
60
%������c2��
��
c3��a=
=
b ��ѡ�������������=��������2��20s��������������Ũ��c1=
0.070
0.070
mol?L-1����0s��20s��������������ƽ����Ӧ����Ϊ0.0015
0.0015
mol?��L?s��-1����3��������ͬ���������������������Ƕ�����������Ҫ�ﵽ����ͬ����ƽ��״̬��������������ʼŨ����
0.200
0.200
mol?L-1����������1���ɱ���֪��60sʱ��Ӧ��ƽ�⣬���ݷ���ʽ�����c��N2O4��������ת���ʼ���ƽ��ʱN2O4��ת���ʣ�
���ݷ���ʽ���㣬����c2��c3���ݴ˽��
60s��Ӧ��ƽ�⣬��Ӧ��������ֵ�Ũ�Ȳ��䣮
��2���ɡ�c��NO2�������ݷ���ʽ�����c��N2O4����20s��������������Ũ��=��ʼŨ��-��c��N2O4����
����v=
����v��N2O4����
��3���ﵽ����ͬ����ƽ��״̬��Ϊ��Чƽ�⣬����ѧ���������㵽N2O4һ�ߣ�����c��N2O4��Ϊ0.100mol/L��
���ݷ���ʽ���㣬����c2��c3���ݴ˽��
60s��Ӧ��ƽ�⣬��Ӧ��������ֵ�Ũ�Ȳ��䣮
��2���ɡ�c��NO2�������ݷ���ʽ�����c��N2O4����20s��������������Ũ��=��ʼŨ��-��c��N2O4����
����v=
| ��c |
| ��t |
��3���ﵽ����ͬ����ƽ��״̬��Ϊ��Чƽ�⣬����ѧ���������㵽N2O4һ�ߣ�����c��N2O4��Ϊ0.100mol/L��
����⣺��1���ɱ���֪��60sʱ��Ӧ��ƽ�⣬c��NO2��=0.120mol/L��
N2O4?2 NO2��
Ũ�ȱ仯��0.06mol/L 0.120mol/L
����ƽ��ʱN2O4��ת����Ϊ
��100%=60%��
c3=0.1mol/L-0.06mol/L=0.04mol/L��
�ɱ���֪��40sʱ��c��N2O4��=0.050mol/L��
N2O4?2 NO2��
Ũ�ȱ仯����0.1-0.05��mol/L 0.10mol/L
����c2=0.10mol/L
����c2��c3��
60s��Ӧ��ƽ�⣬��Ӧ��������ֵ�Ũ�Ȳ��䣬����a=b��
�ʴ�Ϊ��60������=
��2���ɱ���֪��20sʱ��c��NO2��=0.060mol/L������
N2O4?2 NO2��
Ũ�ȱ仯��0.03mol/L 0.060mol/L
����20s��������������Ũ��c1=0.1mol/L-0.03mol/L=0.07mol/L��
��0s��20s��������������ƽ����Ӧ����Ϊv��N2O4��=
=0.0015mol?��L?s��-1��
�ʴ�Ϊ��0.07mol/L��0.0015mol?��L?s��-1��
��3���ﵽ����ͬ����ƽ��״̬��Ϊ��Чƽ�⣬����ѧ���������㵽N2O4һ�ߣ�����c��N2O4��Ϊ0.100mol/L��
���� N2O4?2 NO2��
0.1mol/L 0.20mol/L
�ʴ�Ϊ��0.20mol/L
N2O4?2 NO2��
Ũ�ȱ仯��0.06mol/L 0.120mol/L
����ƽ��ʱN2O4��ת����Ϊ
| 0.06mol/L |
| 0.1mol/L |
c3=0.1mol/L-0.06mol/L=0.04mol/L��
�ɱ���֪��40sʱ��c��N2O4��=0.050mol/L��
N2O4?2 NO2��
Ũ�ȱ仯����0.1-0.05��mol/L 0.10mol/L
����c2=0.10mol/L
����c2��c3��
60s��Ӧ��ƽ�⣬��Ӧ��������ֵ�Ũ�Ȳ��䣬����a=b��
�ʴ�Ϊ��60������=
��2���ɱ���֪��20sʱ��c��NO2��=0.060mol/L������
N2O4?2 NO2��
Ũ�ȱ仯��0.03mol/L 0.060mol/L
����20s��������������Ũ��c1=0.1mol/L-0.03mol/L=0.07mol/L��
��0s��20s��������������ƽ����Ӧ����Ϊv��N2O4��=
| 0.03mol/L |
| 20s |
�ʴ�Ϊ��0.07mol/L��0.0015mol?��L?s��-1��
��3���ﵽ����ͬ����ƽ��״̬��Ϊ��Чƽ�⣬����ѧ���������㵽N2O4һ�ߣ�����c��N2O4��Ϊ0.100mol/L��
���� N2O4?2 NO2��
0.1mol/L 0.20mol/L
�ʴ�Ϊ��0.20mol/L
���������黯ѧƽ����йؼ��㡢��Чƽ��ȣ��Ѷ��еȣ�ע�����֪ʶ�Ļ������գ�

��ϰ��ϵ�д�
�����Ŀ
��100��ʱ����0.40molNO2�������2L�ܱ������У�ÿ��һ��ʱ��Ը����������ʽ��в������õ����������±���
|
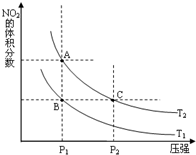 �����γɶ����������NO��NO2��N2O4�ȣ�NO2��N2O4�����ת����
�����γɶ����������NO��NO2��N2O4�ȣ�NO2��N2O4�����ת������1���Է�Ӧ2NO2��g��?N2O4��g����H=-57.2kJ?mol-1
�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��T1
��A��C���������ƽ����Է���������A
��2����100��ʱ����0.40mol��NO2�������2L���ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ�������ݣ�
| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n��NO2��/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n��N2O4��/mol | 0.00 | 0.05 | n2 | 0.08 | 0.08 |
�ڸ�������ƽ�ⳣ��K��ֵΪ
���������������䣬���м��ܼӿ�����Ӧ�����������NO2ת���ʵĴ�ʩ��
��������ͬ����������������������N2O4���壬Ҫ�ﵽ����ͬ����ƽ��״̬��N2O4����ʼ�����ʵ�����
���������������䣬ֻ��������Ϊ�������ĺ�ѹ��������ƽ��ʱN2O4����
 ��100��ʱ����0.40mol���������������2L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±����ݣ�
��100��ʱ����0.40mol���������������2L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±����ݣ�
 �ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�
�ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�