��Ŀ����
��16�֣�. ��ij���塢���Ļ�ɫ��Һ�У����ܺ���NH4+��Fe3+��Ba2+��Al3+��SO42-��HCO3-��I- Cl-���ӡ���������ʵ�飨�����Լ�����������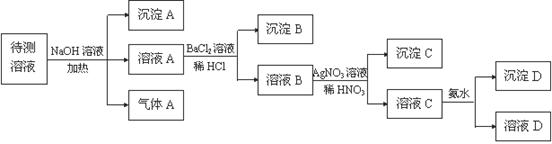
(1) ���� A�Ļ�ѧʽ�� ������A�Ļ�ѧʽ�� ��
(2)����Һ��һ������ ��һ�������� _______ �����ӷ���ʽ��ʾ��������һ�������ڵ�ԭ�� ��
(3)д����ҺC�������ˮ��Ӧ�����ӷ���ʽ ��
(4)������A������A������D�����ʵ�����Ϊ1mol,��SO42-�����ʵ���Ϊ�� mol
��1��Fe(OH)3 (1��) NH3��1�֣���2�� ��2�֣�
��2�֣�
����

��ϰ��ϵ�д�
�����Ŀ

