��Ŀ����
����Ŀ��ij�л�����C��H��O����Ԫ����ɣ������к���8��ԭ�ӣ�1mol���л��ﺬ��46mol���ӣ���ȫȼ�ո��л�������ͬ�����²ⶨCO2��ˮ���������Ϊ2:1��ȡ2��7g���л���ǡ����30mL 1mol/L��̼������Һ��ȫ��Ӧ������д����Ҫ�ļ�����̣���
��1���л������ʽ��
��2���л���ṹ��ʽ��
��3��д�����л�����һ�����������Ҷ�����Ӧ������Ԫ��״������ķ���ʽ��
���𰸡���1��C2H2O4����2��HOOC��COOH����3��HOOC��COOH+HOH2C��CH2OH![]()
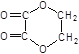 +2H2O��
+2H2O��
�������������������1������CO2��ˮ���������Ϊ2:1�ã�n(C)��n(H)="1:1" ������л���ķ���ʽΪCxHxOy��x��1��y��1��2x+y=8�������ۣ���x=1��y=6��������������46������������
��x=2��y=4����������=6��2+1��2+8��4=46���������⡣
����л���ķ���ʽΪC2H2O4����2�����л������Է�������Ϊ90���ܺ�̼���Ʒ�Ӧ��˵�����л����к����Ȼ���2��7g���л�������ʵ���Ϊ0��03mol��̼���Ƶ����ʵ���Ϊ0��03mol���Ƴ����л��ﺬ�����Ȼ����ṹ��ʽHOOC��COOH����3���������л����������Ӧ������������Ӧʵ�ʣ��䷴Ӧ����ʽΪ��HOOC��COOH+HOH2C��CH2OH![]()
 +2H2O��
+2H2O��







