��Ŀ����
A��B��C��D��E���ֶ�����Ԫ�أ�ԭ���������ε�����Aԭ���γɵ���������һ�����ӣ�Bԭ�ӵ������������Ǵ�����2����Cԭ�ӵ�������������Bԭ�ӵĺ������������ȣ�D��ͬ����Ԫ����ԭ�Ӱ뾶���E��C��ͬ����Ԫ�أ�C��D����Ԫ�ؿ��γɻ�����ף����м������Ӽ����й��ۼ���A��B��C��D����Ԫ�ؿ��γɻ������ң��ҷ���ˮ��������Һ�Լ��ԣ���ش���1��������ĵ���ʽΪ______���ҵĻ�ѧʽ�кܶ��֣�����������______����Է���������С���л�����______��
��2������A2��C2��A��C��D�γɵ����ӻ������ˮ��Һ���γ�ȼ�ϵ�أ��������ĵ缫��ӦʽΪ______������ܷ�Ӧ�õ�1.8g����ʱ���������·ijһ�����ĵ�����Ϊ______��
��3��A��B���γ������ֻ���������ܶ���С����̬������ȼ����Ϊ890.3 kJ/mol���û�����ȼ�յ��Ȼ�ѧ����ʽΪ______��
��4������A��C��D��E�γɵ����ֻ�����������Ӧ�������ӷ���ʽΪ______��
���𰸡�������Aԭ���γɵ���������һ�����ӣ���AΪHԪ�أ�Bԭ�ӵ������������Ǵ�����2����ӦΪCԪ�أ�Cԭ�ӵ�������������Bԭ�ӵĺ������������ȣ�ӦΪOԪ�أ�D��ͬ����Ԫ����ԭ�Ӱ뾶���ӦΪNaԪ�أ�E��C��ͬ����Ԫ�أ���EΪSԪ�أ�C��D����Ԫ�ؿ��γɻ�����ף����м������Ӽ����й��ۼ������ΪNa2O2��A��B��C��D����Ԫ�ؿ��γɻ������ң��ҷ���ˮ��������Һ�Լ��ԣ�����������ΪNaHCO3��CH3COONa�ȣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ����Ԫ�ض�Ӧ�ĵ��ʡ�����������ʽ����⣮
����⣺Aԭ���γɵ���������һ�����ӣ���AΪHԪ�أ�Bԭ�ӵ������������Ǵ�����2����ӦΪCԪ�أ�Cԭ�ӵ�������������Bԭ�ӵĺ������������ȣ�ӦΪOԪ�أ�D��ͬ����Ԫ����ԭ�Ӱ뾶���ӦΪNaԪ�أ�E��C��ͬ����Ԫ�أ���EΪSԪ�أ�
��1��O��Na����Ԫ�ؿ��γɻ�����ף����м������Ӽ����й��ۼ������ΪNa2O2������ʽΪNa+[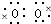 ]2-Na+��A��B��C��D����Ԫ�ؿ��γɻ������ң��ҷ���ˮ��������Һ�Լ��ԣ�����������ΪNaHCO3��CH3COONa�ȣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ�����NaHCO3Ϊ���CH3ONaΪ��Է���������С���л��
]2-Na+��A��B��C��D����Ԫ�ؿ��γɻ������ң��ҷ���ˮ��������Һ�Լ��ԣ�����������ΪNaHCO3��CH3COONa�ȣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ�����NaHCO3Ϊ���CH3ONaΪ��Է���������С���л��
�ʴ�Ϊ��Na+[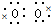 ]2-Na+��NaHCO3��CH3ONa��
]2-Na+��NaHCO3��CH3ONa��
��2������A2��C2��A��C��D�γɵ����ӻ������ˮ��Һ�γɵ�Ϊ��������ȼ�ϵ�أ���ع���ʱ������������ԭ��Ӧ���缫��ӦʽΪO2+2H2O+4e-�T4OH-������ܷ�ӦΪ2H2+O2=2H2O��n��H2O��=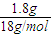 =0.1mol��ת�Ƶĵ��ӵ����ʵ���Ϊ0.2mol������ӵĸ���Ϊ0.2NA��1.204×1023��
=0.1mol��ת�Ƶĵ��ӵ����ʵ���Ϊ0.2mol������ӵĸ���Ϊ0.2NA��1.204×1023��
�ʴ�Ϊ��O2+2H2O+4e-�T4OH-��1.204×1023����0.2NA����
��3��A��B�γɵ��ܶ���С�Ļ�����ΪCH4������ȼ���ȿ�֪�Ȼ�ѧ����ʽΪCH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3 kJ?mol-1��
�ʴ�Ϊ��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3 kJ?mol-1��
��4������A��C��D��E�γɵ����ֻ�����������Ӧ�������ֻ�����ֱ�ΪNaHSO4��NaHSO3�����߷�Ӧ�����ӷ���ʽΪH++HSO3-�TSO2��+H2O��
�ʴ�Ϊ��H++HSO3-�TSO2��+H2O��
�����������ڿ���Ԫ�ص��ƶϵĻ����Ͻ�һ������绯ѧ����ѧ��Ӧ�������Լ����ӷ�Ӧ�����⣬��Ŀ�Ѷ��еȣ������״���Ϊ����л���CH3ONa��ע�ⲻҪд�ɼ����ƣ�
����⣺Aԭ���γɵ���������һ�����ӣ���AΪHԪ�أ�Bԭ�ӵ������������Ǵ�����2����ӦΪCԪ�أ�Cԭ�ӵ�������������Bԭ�ӵĺ������������ȣ�ӦΪOԪ�أ�D��ͬ����Ԫ����ԭ�Ӱ뾶���ӦΪNaԪ�أ�E��C��ͬ����Ԫ�أ���EΪSԪ�أ�
��1��O��Na����Ԫ�ؿ��γɻ�����ף����м������Ӽ����й��ۼ������ΪNa2O2������ʽΪNa+[
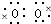 ]2-Na+��A��B��C��D����Ԫ�ؿ��γɻ������ң��ҷ���ˮ��������Һ�Լ��ԣ�����������ΪNaHCO3��CH3COONa�ȣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ�����NaHCO3Ϊ���CH3ONaΪ��Է���������С���л��
]2-Na+��A��B��C��D����Ԫ�ؿ��γɻ������ң��ҷ���ˮ��������Һ�Լ��ԣ�����������ΪNaHCO3��CH3COONa�ȣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ�����NaHCO3Ϊ���CH3ONaΪ��Է���������С���л���ʴ�Ϊ��Na+[
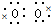 ]2-Na+��NaHCO3��CH3ONa��
]2-Na+��NaHCO3��CH3ONa����2������A2��C2��A��C��D�γɵ����ӻ������ˮ��Һ�γɵ�Ϊ��������ȼ�ϵ�أ���ع���ʱ������������ԭ��Ӧ���缫��ӦʽΪO2+2H2O+4e-�T4OH-������ܷ�ӦΪ2H2+O2=2H2O��n��H2O��=
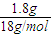 =0.1mol��ת�Ƶĵ��ӵ����ʵ���Ϊ0.2mol������ӵĸ���Ϊ0.2NA��1.204×1023��
=0.1mol��ת�Ƶĵ��ӵ����ʵ���Ϊ0.2mol������ӵĸ���Ϊ0.2NA��1.204×1023���ʴ�Ϊ��O2+2H2O+4e-�T4OH-��1.204×1023����0.2NA����
��3��A��B�γɵ��ܶ���С�Ļ�����ΪCH4������ȼ���ȿ�֪�Ȼ�ѧ����ʽΪCH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3 kJ?mol-1��
�ʴ�Ϊ��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3 kJ?mol-1��
��4������A��C��D��E�γɵ����ֻ�����������Ӧ�������ֻ�����ֱ�ΪNaHSO4��NaHSO3�����߷�Ӧ�����ӷ���ʽΪH++HSO3-�TSO2��+H2O��
�ʴ�Ϊ��H++HSO3-�TSO2��+H2O��
�����������ڿ���Ԫ�ص��ƶϵĻ����Ͻ�һ������绯ѧ����ѧ��Ӧ�������Լ����ӷ�Ӧ�����⣬��Ŀ�Ѷ��еȣ������״���Ϊ����л���CH3ONa��ע�ⲻҪд�ɼ����ƣ�

��ϰ��ϵ�д�
�����Ŀ






 Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������
Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������