��Ŀ����
��10�֣������£�ij������Ũ���ᡢŨ���ᡢ��ͭ��ˮ�Ϳ���Ϊԭ�ϣ������ͼ��ʾ��ȡ����ͭ����(CuSO4��5H2O)��������������̡�

�ش��������⣺
��1����I������ȡ����ͭ���ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��2����������Ӧ�п��Ա�ѭ�����õ�����Ϊ ��д��ѧʽ����
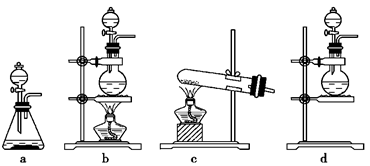
��3����ijͬѧ��ʵ������ģ��������������ʵ����������������ݸ�ͬѧ��˼·������װ����ѡ���ʵ���װ�ã��������ǵı�����뷽���ڡ�
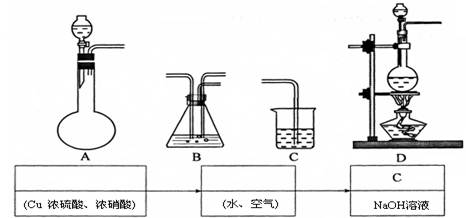
��װ��C�������� ��
��4������ͭ����Ҳ����ֱ����Ũ����ʹ�ͭ��Ӧ��ȡ��������������ȣ���ȱ���� ��

�ش��������⣺
��1����I������ȡ����ͭ���ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��2����������Ӧ�п��Ա�ѭ�����õ�����Ϊ ��д��ѧʽ����
��3����ijͬѧ��ʵ������ģ��������������ʵ����������������ݸ�ͬѧ��˼·������װ����ѡ���ʵ���װ�ã��������ǵı�����뷽���ڡ�

��װ��C�������� ��
��4������ͭ����Ҳ����ֱ����Ũ����ʹ�ͭ��Ӧ��ȡ��������������ȣ���ȱ���� ��
��1��Cu+2HNO3+H2SO4=CuSO4+2NO2��+2H2O ��2�֣�
��2��HNO3��NO2ҲӦ���� ��2�֣�
��3����A B ��2�֣�
������ʣ���NO2 ��2�֣�
��4��Ũ������Cuֱ�ӷ�Ӧ��Ҫ���ȣ�Ũ���������SO2�ڴ�ʵ��������ѭ������ԭ�������ʵͲ���Ⱦ������
��2��HNO3��NO2ҲӦ���� ��2�֣�
��3����A B ��2�֣�
������ʣ���NO2 ��2�֣�
��4��Ũ������Cuֱ�ӷ�Ӧ��Ҫ���ȣ�Ũ���������SO2�ڴ�ʵ��������ѭ������ԭ�������ʵͲ���Ⱦ������
��1��Cu��HNO3��Ũ����Ӧ��ֻ��2molHNO3����ԭ����2molH�� ��H2SO4�ṩ��Cu+2HNO3+H2SO4=CuSO4+2NO2��+2H2O; ��2��HNO3��NO2ҲӦ���֣�3����ͭ��Ũ���ᷴӦ����Ҫ���ȣ���ѡA����Ӧ��Ҫ���������ѡ B��װ��C������������ʣ���NO2����ֹ��Ⱦ������4��Ũ������Cuֱ�ӷ�Ӧ��Ҫ���ȣ�Ũ���������SO2�ڴ�ʵ��������ѭ������ԭ�������ʵͲ���Ⱦ������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
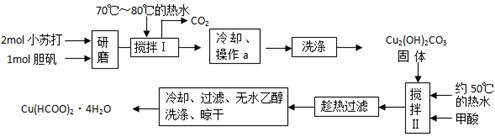
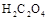 ���ֽ�Ļ�ѧ����ʽΪ��
���ֽ�Ļ�ѧ����ʽΪ��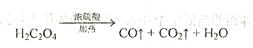
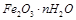 ��
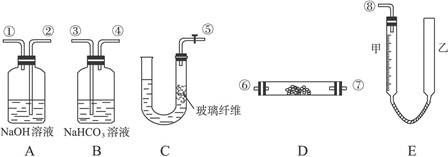
��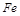 ���ֳɷݣ������ò���ֽ������CO�����ⷴӦ��ʵ��װ������ͼ��ʾ��
���ֳɷݣ������ò���ֽ������CO�����ⷴӦ��ʵ��װ������ͼ��ʾ��
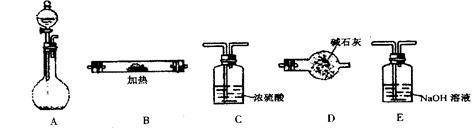
 ������ ��
������ �� ����˳���ǣ�����ĸ��������������
����˳���ǣ�����ĸ�������������� ������������E
������������E l2��2H2SO4
l2��2H2SO4 2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣
2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣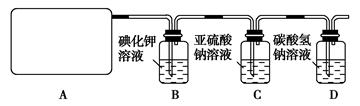
 ________________ ��
________________ ��