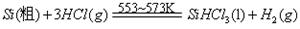��Ŀ����
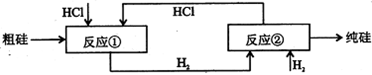
±ˮ�е���Ҫ�ɷ�Ϊʳ�Σ��������������ʽ�ΪNaBr����Ϊ�˳��������Դ�����о���Ա��������ɹ��±ˮ�ͻ���̼�����Ϊԭ����ȡ����Ĺ��գ���������������ͼ��

��1��д��±ˮ��̼����立�Ӧ�Ļ�ѧ����ʽ_______________��
��2������I��_________����������ƣ����Լ�XΪ________����Һ��NH4Cl�⣬������_________��
��3���������к���NH4Cl���ʣ�����ܵ��´����л������ʣ�д������ʱ�������ʵĸ���Ӧ�Ļ�ѧ����ʽ__________________��
��4��ʵ���Ҽ���������Ƿ���NH4Cl�IJ�����_________________��
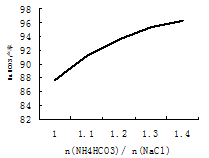
��5��̼��������Ȼ��Ƶ����ϱ���Ӱ�촿������������֮һ����ͼ��(Ħ����)��NaHCO3���ʵ�Ӱ�졣ʵ��������ѡ��=1.2��
���������˵����ȷ����___________��
a��̼����粒�����Ϊ�˳������±ˮ
b��̼����粒�����Ϊ�˼��ٴ����е�NaCl
c��̼����粒���̫�ཫ�ᵼ�������ɱ�����
�ڲ��������������е���ģ�����1mol���������̼�����________mol��


��1��д��±ˮ��̼����立�Ӧ�Ļ�ѧ����ʽ_______________��
��2������I��_________����������ƣ����Լ�XΪ________����Һ��NH4Cl�⣬������_________��
��3���������к���NH4Cl���ʣ�����ܵ��´����л������ʣ�д������ʱ�������ʵĸ���Ӧ�Ļ�ѧ����ʽ__________________��
��4��ʵ���Ҽ���������Ƿ���NH4Cl�IJ�����_________________��
��5��̼��������Ȼ��Ƶ����ϱ���Ӱ�촿������������֮һ����ͼ��(Ħ����)��NaHCO3���ʵ�Ӱ�졣ʵ��������ѡ��=1.2��
���������˵����ȷ����___________��
a��̼����粒�����Ϊ�˳������±ˮ
b��̼����粒�����Ϊ�˼��ٴ����е�NaCl
c��̼����粒���̫�ཫ�ᵼ�������ɱ�����
�ڲ��������������е���ģ�����1mol���������̼�����________mol��
��1��NaCl +NH4HCO3 �� NaHCO3�� + NH4Cl��2�֣�
��2�����ˡ�ϴ�ӣ�2�֣� ���ᣨ1�֣� NH4Br����NaBr����1�֣���3��NaHCO3+ NH4Cl�� NaCl +NH3��+H2O + CO2����2�֣�
��4��ȡϴ��Һ������������������Һ�����ԣ��ٵμ���������Һ������˵���������𰸺������ɣ�1�֣�
��5���� bc��2�֣� �� 2.4mol��1�֣�
|
��4��ȡϴ��Һ������������������Һ�����ԣ��ٵμ���������Һ������˵���������𰸺������ɣ�1�֣�
��5���� bc��2�֣� �� 2.4mol��1�֣�
��

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ

 2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�����������ҡ���������ʹ�ô��� ��Ӧ�Ħ�H���������С�����ı䡱����
2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�����������ҡ���������ʹ�ô��� ��Ӧ�Ħ�H���������С�����ı䡱����
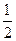 H2��g��+
H2��g��+ H="_____________" kJ��mol-1��
H="_____________" kJ��mol-1��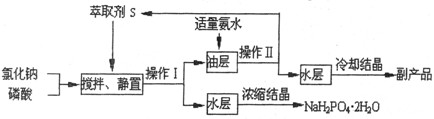
 NaH2PO4��HCl
NaH2PO4��HCl 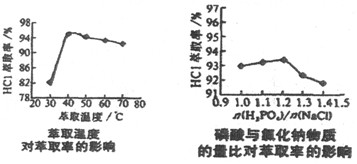
 3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺ H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K
H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K
 CO2��2H2O��4Cu����������ȫ��Ӧ��Ӳ�ʲ����ܵ���������4.8g������Ӧ�����������ͨ�������ij���ʯ��ˮ��������գ����ɳ���8.5g��
CO2��2H2O��4Cu����������ȫ��Ӧ��Ӳ�ʲ����ܵ���������4.8g������Ӧ�����������ͨ�������ij���ʯ��ˮ��������գ����ɳ���8.5g��
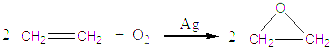
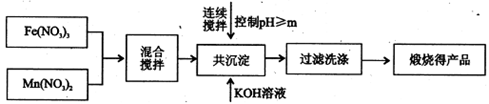
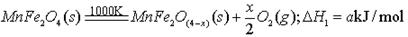
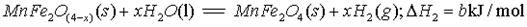
 (����ĸ���)��
(����ĸ���)�� B�����Ͽ�ѭ��ʹ��
B�����Ͽ�ѭ��ʹ��